The role of tissue and cellular stiffening in maladaptive vascular smooth muscle cell phenotypic change.
Cardiovascular disease (CVD) refers to a heterogeneous group of diseases afflicting the heart and vasculature with similar underlying pathophysiological manifestations, such as coronary heart diseases, cerebrovascular disease, hypertension, peripheral arterial disease, congenital heart diseases and heart failure. The prevalence of CVD had reached global epidemic proportions, with an estimated 422.7 million cases worldwide [Figure 1]1. CVD is the universal leading cause of mortality, resulting in 17.9 million deaths in 2015, accounting for 44% of all non-communicable disease deaths2. In the UK, around 7 million people are living with CVD and 25% of all deaths (>150,000 deaths/annum) are attributable to CVD3.
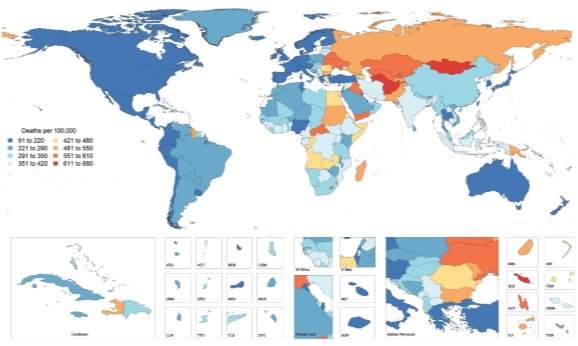
Figure 1: Global Map of Age-Standardised Death Rate of CVD in 2015. Choropleth map illustrating the estimated age-standardized mortality rate of total CVD in 2015 for each country1.
The worldwide burden of CVD on public health is escalating due to global demographic and socio-economic trends, particularly population ageing, economic growth in Asia and the obesity epidemic observed in developed countries. In addition, more than 75% of CVD related deaths occur in low- and middle-income countries, as a consequence of restricted access to primary healthcare and affordable treatments. Thus, an enhanced understanding of cardiovascular physiology and CVD mechanisms, as well as the development of novel, more targeted and low-cost prevention, early diagnostic and therapeutic strategies are required to reduce the incidence of CVD mortality4.
CVD pathogenesis and related complications are attributed to numerous risk factors including metabolic factors like hypertension, hyperglycaemia, dyslipidaemia and obesity, coupled with behavioural factors such as imbalanced diet, physical inactivity, excessive alcohol consumption and tobacco use. Ageing is an integral, independent risk factor for CVD5, however many questions are unanswered concerning ageing and CVD. Here, the focus will be on elucidating possible links between ageing, CVD and vascular calcification.
Arterial compliance is dependent upon intrinsic material stiffness and the arterial architecture. Arterial stiffness can be defined as a diminishing distensibility that characterises the relative changes occurring in the lumen cross-sectional area for a given variation in blood pressure18.
The distensibility or stiffness of the arteries contributes to wave propagation and reflection in the arterial vasculature, where the arterial pulse disseminates with a certain speed known as the pulse wave velocity (PWV), continuously varying in amplification and shape. Arterial stiffness has aetiological implications in various cardiovascular diseases and has been identified as a key independent risk factor for all-cause and CVD mortality19,20. Aortic PWV is a reference parameter largely accepted as the simplest, non-invasive, accurate and reproducible measurement of central arterial stiffness for large elastic arteries. Clinically, the carotid-femoral PWV is considered as the ‘gold-standard’ measurement to determine arterial stiffness, measured along the aorto-iliac pathway and has been previously utilised to determine the predictive value of aortic stiffness and cardiovascular incidence21. Arterial stiffness indexes, for example, Young’s elastic modulus calculated from stress-strain curves, are utilised for smaller-sized muscular arteries. Both central and peripheral stiffness is influenced by the intricate interactions of the intramural cells and ECM, which are responsible for the mechanical function and structural integrity of the vasculature, differing with vessel size22. The aetiology of arterial stiffening is attributed to various molecular and cellular determinants including key ECM components (primarily elastin and collagen fibres), cell-ECM interactions and VSMC tone regulatory proteins. In addition, mechanical factors of arterial wall remodelling (shear stress and pulsatile, circumferential stress) and VSMC plasticity are key determinants of arterial stiffness18.
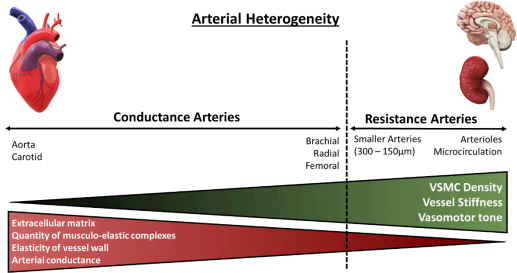
Figure 2: Architectural and functional divergence of the arterial vasculature. The diversity of the arterial vasculature ensures that conductance and resistance arteries can exert their respective functions. Larger elastic arteries, known as conductance arteries, convert pulsatile flow and pressure into continuous pressure, enabling oxygen delivery with nominal energy loss in arteriolar vascular walls. Resistance arteries are smaller in diameter, include arterioles and the microcirculation, and are responsible for the distribution of blood flow to target organs. Red triangle: Demonstrates arterial compliance of various arteries, amount of ECM present within vessels’ wall and number of musculo-elastic complexes. Green triangle: Demonstrates density of VSMCs within arterial wall, vessel stiffness and vasomotor tone, the function of which augments with diminishing vessel size. Adapted from Lacolley, P. et al (2017)14.
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more