The demand for DNA vaccines in large quantities at high purity for gene therapy is on the increase. As it helps to stimulate antibodies production in human and provide immune protection against many diseases such as cancer, malaria, HIV and other diseases (Laere et al., 2016) and have potential advantages over conventional vaccines.
Therefore, this report covers a detailed design and cost (to an accuracy of +/- 20%) for a new manufacturing facility to produce DNA vaccines to be built on a greenfield site. Applied current good manufacturing practice (cGMP) and complied with all the regulatory guidelines set up by various agencies.
A process to produce commercial pDNA covers: Fermentation, recovery and product purification. The layout of the manufacturing facilities has been designed in a way to allow a good waste, raw material and personnel flow to minimise the risk and contamination. All of these are an example of current good manufacturing practices which are vital for the production of the therapeutic product (DNA vaccines).
Plant services, system and utilities has been designed to meet the requirement for the manufacturing facility which includes Water for CIP to clean equipment and media preparation, Clean steam for SIP to sterilise the equipment, Heating, ventilation and air conditioning (HVAC), water, Compressed air, Effluent treatment, Nitrogen, USP, CIP system, refrigeration and more. (Cole, 1998).
The facility will take up to 2.6 years to complete and qualify (GSK, 2017). The project schedule will depend on various activities these must be completes to produce DNA vaccine facility, ready for start-up. These includes Front end design, Detailed design, Procurement of equipment, construction, recruitment of staff, commissioning of facility and validation of process. (Adkin, 1998)
Finally, the costs of the pharmaceutical plant are a serious consideration when planning whether a plant will be commissioned or not. The economic evaluation for the DNA Vaccine Plant suggests that the plant will be at significant profit with a payback time of less than 2 years.
Contents
3.0 Design of Manufacturing facility
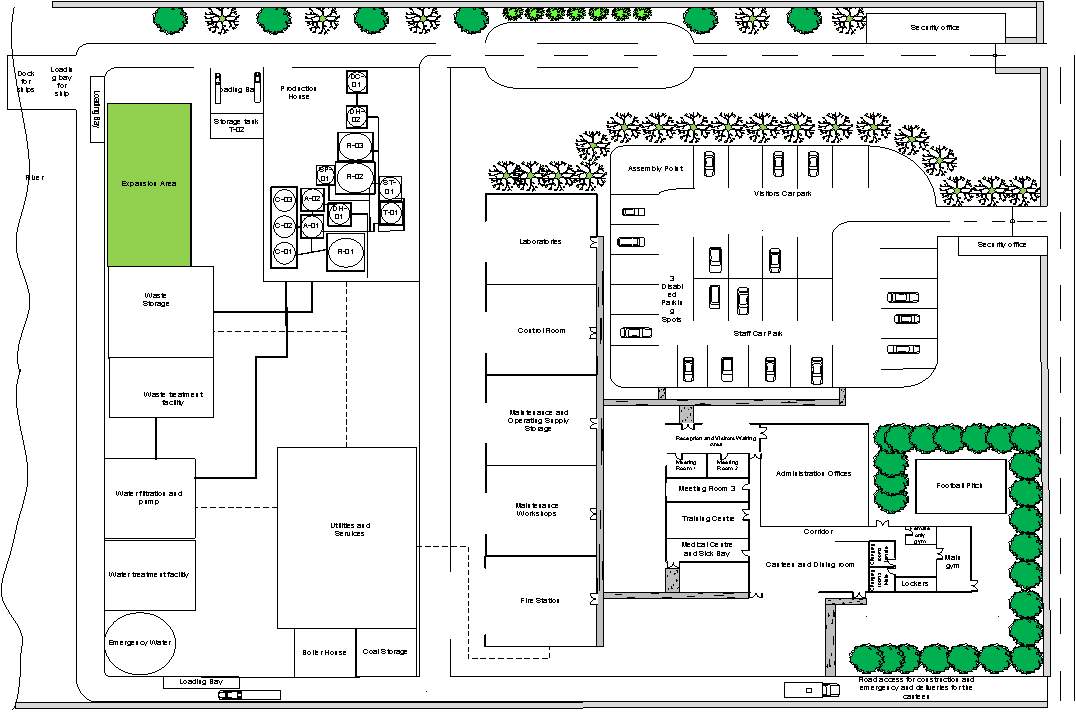
3.21 Layout of the Plant site appendix 1
3.22 Layout of the Production House appendix 2
3.4 Room Specification data sheets appendix 3
4.0 Plant Services, Systems and Utilities
4.1 Heating, ventilation and air-conditioning (HVAC) system
4.7 Process control & instrumentation
5.0 Process Validation and cGMP
Appendix 1 Layout of the Plant site
Layout of the Production House Appendix 2
Plant design and manufacturing principles in DNA vaccine production
DNA Vaccines helps to stimulate antibodies production in human and provide immune protection against many diseases such as cancer, malaria, HIV and other diseases (Laere et al., 2016) and have potential advantages over conventional vaccines. The demand for DNA vaccines in large quantities at high purity for gene therapy is on the increase. However, inaccessibility of DNA vaccines for the treatment of diseases can cause several problems. Therefore, to meet the rising demand for DNA vaccines, there is a need for designing & manufacturing facilities to produce DNA vaccines. In order to fulfil the need for the production of DNA vaccines the manufacturing facility such as design, operation and layout must comply with the regulatory requirements stipulated that the production of DNA vaccine must be performed under current good manufacturing practices (cGMP) and good design practices (GDP). Also, comply with the guidance regarding the specification, quality testing and manufacturing standard of DNA products by regulatory agencies such as the US Food and Drug Administration (FDA) and the European Agency for the Evaluation of Medicinal Products (EMEA) and also regulation stipulated by the country where the facility is being constructed. (Przybylowski et al., 2017)
According to the EU guide, GMP is the part of Quality Assurance (QA) which make sure that products are consistently produced and controlled to the quality standards appropriate to their intended use and as required by marketing authorisation or product specification (Institution of Chemical Engineers (Great Britain), 2003).
When designing a new drug production manufacturing facility, the following consideration needs to be made (GMP requirements). To ensure the safety, identity, purity and potency of the manufactured product (Przybylowski et al., 2017). Not placing the patient at risk due to insufficient safety or quality.
A process of commercial pDNA manufacturing includes: Fermentation, recovery and product purification. Below figure 1 shows the unit operation involved in the manufacturing process.
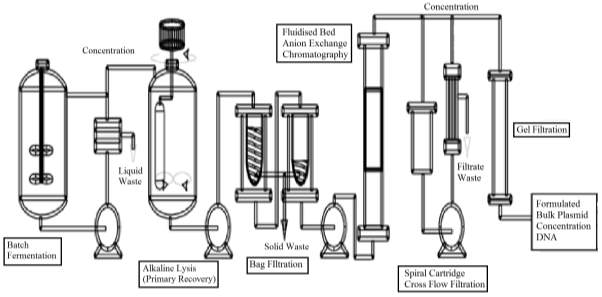
Figure 1 : the process flow of the production of therapeutic plasmid purified bulk products.
Batch Fermentation – Fermentation process is the starting point for the production of pDNA in the bio-reactor, and the aim is to maximise pDNA productivity per unit cell mass. This takes place under the optimum condition to maximise the cell growth. (Adkin, 1998)
Received raw material is placed in quarantine unit until clearance from QA. Production culture stains within the facility are maintained as MCB and WCB’s. At the start, bacteria from a WCB’s vial is revived and grown in a propagation area up to 2 cultures. Sterilised prepared media is feed into the fermenter. Two seed vessel of volume 15L and 150L are used to produce inoculum for the 1000L cell culture vessel. Operated in the fed-batch mode to provide product for successive downstream processing with operating temperature of 37oC optimum for bacteria. Upon the establishment of maximum cell growth. The broth is cooled to 40C and pumped to centrifuge at a flowrate of 1000L/h, as this allows effective separation of cellular material. Concentrated broth contains pDNA and some other cell organelles; this is discharged in the alkaline lysis vessel. (Adkin, 1998)
Alkaline Lysis – three step process. 1) concentrated broth is suspended in the buffer solution for alkaline lysis – low viscosity. 2) The cell suspension is lysed by mixing with NaOH/SDS – solution changes highly viscous. 3) 3M potassium acetate neutralisation causing the precipitation of impurities such as chromosomal DNA whilst minimising the loss of pDNA yield, the solution becoming Newtonian again. Bag Filtration – Disposable non-leaching nylon bags doesn’t contaminate the product, fully scaleable. This partially removes the solid precipitate from the lysate before further product purification.
Anion Exchange chromatography – separation occurs due to electrostatic attraction between the solute and charged dense clusters. Anion exchange chromatography clears all the cationic proteins and lipopolysaccharides and retains all forms of pDNA, the residual cDNA, RNA and any anionic proteins. (Nigel, 2017)
Gel filtration – The removal of small RNA, aggregates or clipped products by Size Exclusion Chromatography; a non-adsorptive chromatography process that separates cluster that differs in size from pDNA in desalting solution. (Nigel, 2017)
Endotoxin Removal – the produced product should be free from all sort of contaminants for the purposes of therapeutic. Therefore, the final step includes the removal of anionic endotoxin by a particular adsorptive chromatographic process. (Nigel, 2017)
Finally, formulating the product by putting stabiliser, correct salt concentration and sterile filtration & lyophilisation.
The following Raw materials are needed for the production of DNA vaccines. All the raw materials entering the process should be controlled and tested to make sure the manufacturing process meet the standard requirements. The most important Raw material is plasmid DNA. Therefore, it should be fully characterised. For Therapeutic pDNA E. coli is the most common host. (Przybylowski et al., 2017)
Raw materials required – pInG human tyrosinase (pING-HT) plasmids, E.coli, Nutrient – Glucose, antifoam,water, liquid nitrogen, ammonia hydroxide, Ethylenediaminetetra acetic acid diammonium salt (EDTA), Hydrochloric Acid, Magnesium Sulphate, Polypropylene Glycol (PPG), Potassium Acetate, Potassium Phosphate, Sodium chloride, Sodium Hydroxide and Tris Hydroxymethyl methylamine (TRIS) buffer. (Adkin, 1998)
The following table shows the manufacturing equipment required for the process, these should be designed, located and maintained to suit its intended purpose and these should not affect the quality of the product.
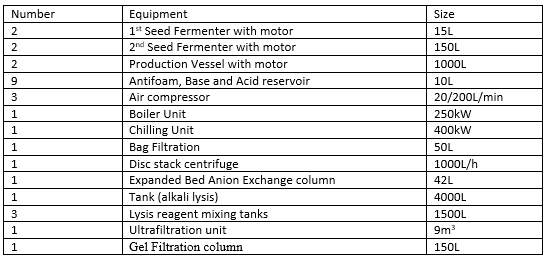

Table1: Equipment used in the process (Adkin, 1998)
The correct manufacturing of the product (DNA Vaccines) depends upon people/staff. Therefore, qualified personnel must carry out all the task which are the responsibility of the manufacturer. The manufacturer should have enough staff with essential qualification and experience, and they should be aware of cGMP and receive appropriate training. (Inspection and Healthcare, 2002)
All the staff members whose activate could affect the quality of the product should be provided with adequate and continuing training such as technical, maintenance and cleaning staff.
Key personnel include Head of Production and Head of Quality Control (QC).
Other personnel include Process Engineer, Technician, Maintenance, Operative, Assistance and cleaning. (Inspection and Healthcare, 2002)
The design of the new manufacturing site for (DNA Vaccine) will be laid out for primary production, secondary production, research and development, warehousing and distribution/administration and head office activities. (ICheme, 2003)
The location of the drug production manufacturing facility is crucial to its profitability as it can be affected by raw material supply, climate, transportation, utilities and services, etc.
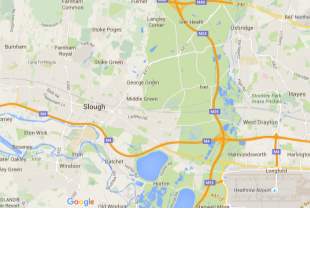
Site selection
The DNA vaccine plant will be located in southern England, Slough. This is chosen because the site is relatively near to greater London which can create huge advantages which include:

The detailed design of the layout of the Plant site and Manufacturing facility are given in the appendix. The layout of the manufacturing facilities will be designed in a way to allow a good waste, raw material and personnel flow to minimise the risk and contamination. All of these are an example of current good manufacturing practices which are vital for the production of the therapeutic product (DNA vaccines). The design of manufacturing facility is divided into three sections which include:
Material/Waste flow
This includes all the movement of materials or waste material. Typical criteria include: (ICheme, 2003)
Raw Materials and supplies flow
Quarantine storage room — Release Storage room (wipe with 70% isopropanol) — Gowning room (wipe with 70% isopropanol) — pDNA production Cleanroom.
Final Product flow
pDNA production cleanrooms — Gowning room —- Final-product storage room
Waste flow
pDNA production cleanrooms —- Gowning room — Biomedical waste disposal
Personnel Flows
This includes the influence personnel have on the quality of the product that might be caused by their contact with the product. The criteria that have been included in the Production House layout include: (ICheme, 2003)
Personnel flow example
Pre-Gowning room to Gowning room (Sterile garments, double gloves) to pDNA production cleanroom.
The layout of the Production house illustrates the Personnel flow, Material flow, waste and equipment flow appendix 2. Different pathways for clean and unclean materials/Personnel to avoid mixing and contamination has been used in the design to comply with cGMP requirements.
Plant services, system and utilities are designed to meet the requirement for the manufacturing facility. DNA Vaccine manufacturing requires following services:
Water for CIP to clean equipment and media preparation, Clean steam for SIP to sterilise the equipment, Heating, ventilation and air conditioning (HVAC), water, Compressed air, Effluent treatment, Nitrogen, USP, CIP system, refrigeration and more. (Cole, 1998)
HVAC system is an important part of the production clean rooms design and layout, ensuring that the product purity and quality are not affected by the room temperature, humidity, pressure, air particles and cross contamination. Providing control/healthy environment for process operation and occupants. (UK, 2015)
HVAC system is managed by automated temperature control system (ATC), which controls the humidity, Air distribution and air quality for the process operation. There various types of HVAC systems which are found in the pharmaceutical facilities and selection depends on the requirements within the plant such as environmental conditions and the level of product containment. The degree of control within the plant increases the complexity and the cost of HVAC. (ICheme, 2003)
The HVAC systems for the DNA vaccine manufacturing facility will include: Separate systems for each work centre and total loss systems to minimise the risk of cross-contamination. Terminal HEPA filters on supply and extract to control dust, microorganisms and airborne particles. Sterile (+) or containment (-) pressure cascades by Airlocks and air handling unit (AHU), Low humidity, Dust extract and specified classification of the clean room. (ICheme, 2003)
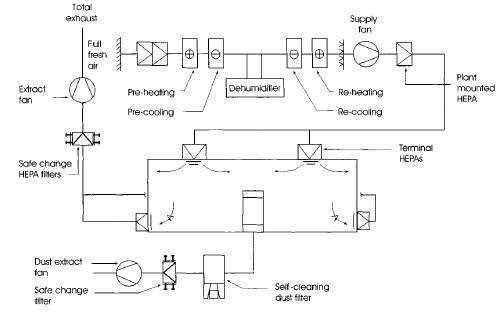
Figure 3: Low humidity total loss containment clean room. (ICheme, 2003)
The DNA vaccine industry comes under the regulations of MCA, FDA and MHRA. They constantly review with great attention the treatment of water in all segments of the industry used for rinsing, washing and product formulation. (Cole, 1998)
Water is considered to be one of the most difficult product to maintain the required standard not only because of the chemical impurities but, the bacterial contamination, as some system contain components which support the growth of bacteria in water (Cole, 1998). DNA vaccine manufacturing facility will use the water provided by the water treatment plant. Town water, process water and wastewater from the plant will be treated to provide other types of water by various processes such as ion Exchange Deionisation (DI), Reverse Osmosis (RO), Electro Deionisation (EDI) and Distillation (WFI). (Cole, 1998) Types of water will be used in the manufacturing facility are:
Town water/portable water – water from the mains and the quality changes throughout the year. Also, known as the drinking water. Microbiological less than 500cfc/ml and no bacterial colonies according to WHO. (ICheme, 2003)
Process Water – this is normally a portable water which has been passed through the site. Used for washing equipment and cooling. This is also an input for the purified stream (ICheme, 2003)
Purified Water – softened and passed through a UV source to kill live bacteria. Preparation of drugs that do not require WFI (non-sterile products), development of clean solution and input for WFI. According to WHO, Microbiological level less than 100cfc/ml and without any pathogens. (Cole, 1998)
Water for injections (WFI) – softened, extremely low bacterial count and reduced endotoxin. Commonly used for the preparation of the sterile drug. This is generated via distillation with some other treatment method (Cole, 1998). Aerobic Bacteria level < 10cfc/ml and Endotoxin < 0.25 I.U./ml.
The town water and the process water will be used in amenities – admin, lobby, drinking and washing equipment and cooling. The purified water and WFI will be utilised in the main production line for producing the drugs. Purified for initial rinsing and WFI for final rinsing. (GSK, 2017)
Purified water process flow for DNA Vaccine Facility
The following process will be used to purify the town/process water for the production of DNA Vaccine.
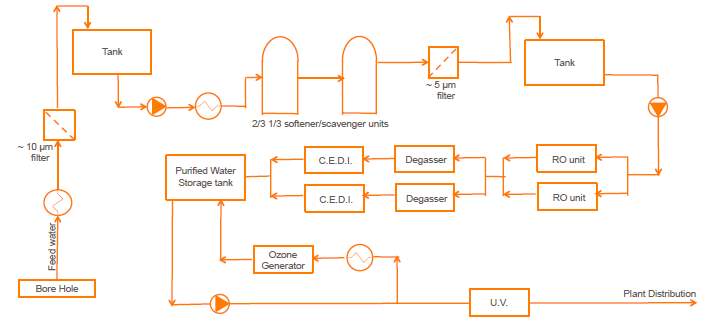
Figure4: Purified water process flow. (Vilhelmsen, 2017)
Clean steam is produced using a steam generator, and it is commonly utilised in a pharmaceutical application where the steam and its condensate are in direct contact with the product to avoid contamination (this is governed by cGMP). (ICheme, 2003)
Clean steam will mainly be used for the sterilisation of process as a sterilising agent; it will also be used in an autoclave and for the humidification for clean rooms. The steam must have the same specification as the water for injections by BP standards for WIF, ensuring the right quality and cleanliness of the steam. This requirement allows the WIF for steam generation. (ICheme, 2003)
Pipeline within the facility can trap condensate which poses the risk of bacterial growth and reduces the effectiveness of sterilisation. Therefore, there should be a steam trap at 30m intervals for efficient removal of condensate. (ICheme, 2003)
Combined heat & power CHP station will be used onsite to provide heat and power supply. CHP is also known as cogeneration simultaneous production of electrical power and heat from natural gas, and it has a heat recovery system, uses the by-product heat which results in higher thermal efficiency over the conventional power station. The overall efficiency of CHP system is greater than 80%. (admin, 2014)
Therefore, the facility will use CHP station onsite to provide heat and power supply. CHP will operate on natural gas, biomass and waste (as a renewable source). The facility will use the excess thermal energy for steam generation and for heating by using pinch technology.
It is critical that the DNA vaccine manufacturing facility to obey with all the GMP requirements. The level of hygiene is one of the primary element, involves cleaning plant items and areas that can affect the purity/quality of the manufactured product. Clean in Place (CIP) and sterilise in Place (SIP) will be used in the facility to minimise product loss, removal of soil from product contact surfaces and elimination of micro-organism to meet the desired hygiene standard. But, validation of the cleaning procedure will be necessary before it can be applied. Once this is approved by the relevant governing body, the maintenance of the cleaning systems should be continuously monitored throughout the cleaning and documented which includes the parameters such as flowrate, time, temperature and detergent.
CIP is a very simple system, consist of a tank filled with the correct concentration of cleaning agent which is heated to a specified temperature by recirculation and then this is pumped into the pipework or vessel followed by drainage. The important consideration here is the control of flow rate, superficial velocity, temperature and the concentration of detergent of cleaning agent. The facility will use decentralised maximum detergent recovery system. The integration of CIP system with process plant to allow unit cleaning as it has great flexibility, efficiency and objects can be cleaned during production. The cleaning will be measured in different ways such as measurement of mass/volume to assure the right amount of CIP fluid gone through; Pressure ensures that fluid is contacting with the surface at the right force and Conductivity to make sure that the chemical is out of the system. (ICheme, 2003) (Goode, 2017)
Function of typical CIP stages
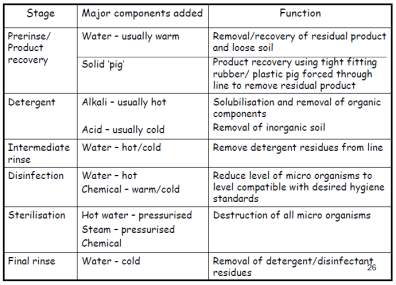
Table 2: Typical function of CIP stages (Goode, 2017)
SIP is not a part of CIP, and this usually comes after the CIP system as detergent cannot clean all part of the plant. The aim of the SIP system is to kill all the viable microorganism (sterilise). Thermal sterilisation (saturated steam sterile) will be used for sterilising the plant equipment which has already been cleaned using CIP system. Similar paraments will be monitored for SIP measurements (Goode, 2017). Steam for cleaning must be pure, and the pressure must be down to 1.2 Barg and temperature 121oC at the point of use.
To produce a sterile product (DNA Vaccine), clean environment is required to minimise the risks of product contamination. In the manufacturing process, people and manufacturing personnel are the biggest sources of contamination, therefore, to reduce the contamination a clean room need to be designed. A clean room is a room in which air supply, air distribution and air filtration are regulated to controls the level of airborne particle concentrates and also meet the cleanliness standards specified by the current standard ISO 14644-1. There is also a specific cleanroom classification for pharmaceutical manufacture figure 4. The table illustrates that the clean rooms are classified into various grades, and it is specified by EU GMP Annex 1. (ICheme, 2003)
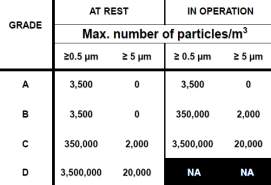
Figure 5: cleanroom categorisation based on max no of particles/m3
There are 4 grades of the clean room.
Grade A – the local zone for high-risk operation, Grade B: for aseptic preparation and filling and grade C & D: for clean areas for carrying out less critical stages in the manufacture of the product. The cleanroom should be monitored regularly during operation in order to control the particulates. (Inspection and Healthcare, 2002).
The manufacturing facility will have clean room from cGMP grade B to grade D as specified in the plant layout figure 2 in appendices. There will be strict environmental measures for all clean room within the facility to control the air supply/distribution, temperature and pressure. (ICheme, 2003)
Cleanroom with cGMP grade C required lots of air at controlled temperature and humidity, the supplied air in the cleanroom will be filtered through highly efficient HEPA filter which can filter 0.3um airborne particulates with the collection efficiency of 99.97%. Cleanroom with grade C, a unidirectional laminar flow air distribution pattern is essential to a vertical air flow of moderate velocity, where the air is fully filtered via HEPA filters at the ceiling. This type of flow is very useful as it is easier to predict particle travel and no dead areas for contamination. AHU system will be also used to recirculate 80% of the air in the cleanroom to reduce particulate contamination with the product. Also, HEPA filtration on exhaust air system located near the point of extract from the room to minimise contamination. (ICheme, 2003)
Air distribution within in the cGMP grade D will be non-directional turbulent flow; this forms air vortex which affects the removal of airborne particles. (ICheme, 2003)
Isolator technology will be used in the facility to reduce human interventions in processing areas; this will reduce the risk of microbial contamination of DNA vaccine from the environment. And, a minimum number of personnel will be present in the cleanroom, and all employee will receive regular training as this is vital during aseptic processing. (Inspection and Healthcare, 2002)
During the manufacturing process of DNA vaccine, certain factors can influence the purity, quality and quantity of the final product. Therefore, effective process and instrumentation control system will be required to maintain the manufacturing process at desired operating conditions, safely and efficiently while satisfying environment and product quality requirement. (GSK, 2017) (AHMED, 2016)
Process and instrumentation control systems monitor and control the process dynamics to function within the set point, set by the operator and the aim of the control loop is to ensure smooth, and rapid return to stable condition following process deviation. Control system also play an important role in plant start-up and shutdown includes alarms and automatic shutdown system this will be integrated part of the system to keep the process variable within safe operating limits. (GSK, 2017) (Ingram, 2017)
The manufacturing facility will use Feedback control, Feedforward control, cascade and Adaptive control as the main control strategy and these will be managed at control room which will be located within the facility. (GSK, 2017)
Feed-forward control will be used in conjunction with the feedback control to measure and correct the effect of process disturbance before they affect the controlled variable. Such as supply of heat to the reactor by a heating jacket. (Ingram, 2017)
Cascade control will also be used. The advantage of having the cascade control is that the secondary measured variable is very close to the potential disturbance. Therefore, cascade control increases the response times, and the potential disturbances are detected rapidly. (GSK, 2017)
Special considerations for DNA products are required such as the flexible operation of process equipment, Batch sequencing /scheduling and avoid cross contaminations. (Ingram, 2017)
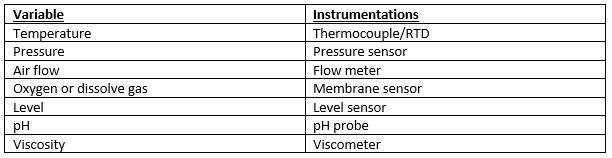
Table 3: Controlling variable and measuring instruments (Ingram, 2017)
The purpose of instrumentation is to monitor key process variable during plant operation in order to facilitate process control, process monitoring & warning and process logging. Instrumentation will be designed to monitor relevant process conditions. Onsite (indicators) or in control room (indicate and control). (GSK, 2017) (Ingram, 2017)
In the facility, certain process requires close monitoring, i.e., the aeration of the fermenter (supply of a correct amount of oxygen is essential for the survival of bacteria) therefore, inline and online instrumentation will be used to control the amount of oxygen as this can affect the final output. (GSK, 2017)
There will be three main type of instrumentation in the facility. In-line (placed in direct contact with the product, level/temperature sensor), on-Line (instrument close to process UV analysis) and off-line (sample is withdrawn from the process for analysis, Filter trains). (Ingram, 2017)
The aim of this manufacturing facility is to produce a safe, pure and efficacious product (DNA vaccine), to meet this objective QA procedure must be in place. cGMP is a part of QA which make sure that products are consistently produced and controlled to quality standards appropriate to their intended use. (ICheme, 2003)
When designing the manufacturing facility, the following consideration needs to be made (GMP requirements). To ensure the safety, identity, purity and potency of the manufactured product (Przybylowski et al., 2017). Not placing the patient at risk due to insufficient safety or quality.
Guideline on general principles of process validation defines validation as ‘Establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its predetermined specifications and quality attributes.‘ (ICheme, 2003)
Validation in the Pharmaceutical industry is an important part of a current good manufacturing practice cGMP; it makes sure that the production facility (DNA vaccine Production facility) is performing adequately to meet its specification and quality attributes. Validation is conducted for three main reasons. (ICheme, 2003)
Validation Plan
All the key elements of validation programme such as process evaluation/identification will be clearly defined and documented and will be presented in the form of validation master plan VMP – which defines the overall project validation approach and the associated rationale. Upon completion of VMP, this document will become a valuable tool to present to the regulatory officials that compliance with regulation has been made. The report will contain detail plan describing procedures that have been placed to assure a validated and compliant manufacturing facility with cGMP. (ICheme, 2003). Typical VMP structure is shown below this differs from project to project.

Figure 6: VMP structure that will be used for DNA manufacturing facility. (ICheme, 2003)
Requirement & Verification of the Design
User requirement specification (URS) – it’s a formal document prepared by the user, record what the user need the design to do, includes specific information on quality, quantity, performance, environment, etc. of the manufacturing product (DNA vaccine). it’s the basis for qualification/validation, and it represents a contract between user and project team. Design specification that meets the user requirement is verified by a process called design qualification (DQ). It will be tough to assess whether the design for (DNA vaccine) is suitable or not without URS. (Cameron, 2017)
Qualifications
Validation master plan defines which system are to be qualified, and the next stage involves qualification of equipment and ancillary system before starting process validation. Qualification is conducted by following activities. (Inspection and Healthcare, 2002)
For the production of DNA vaccine, Prospective Validation is the preferred approached, as this is commonly performed on API products and this should be completed before commercial distribution.
lifecycle maintenance
Validation review is the key part of the lifecycle. Therefore, an annual review will be performed looking at aspects of change control and quality data.
What to validate in the DNA manufacturing facility?
DNA vaccine must meet the minimum product specification set by the authority before releasing.
The designing of the manufacturing facility to produce DNA vaccine requires cautious planning and management. The facility can take up to 4 years to complete and qualify (GSK, 2017). The project schedule depends on various activities these must be completed to produce DNA vaccine facility ready for start-up. These includes Front end design, Detailed design, Procurement of equipment, construction, recruitment of staff, commissioning of facility and validation of the process. (Adkin, 1998)
To be competitive in the market, the manufacturing Plant will be constructed, commissioned and validated as fast as possible. To achieve this target, we will use fast track/alliance approach in creating the project schedule and carrying out dependant activates concurrently. (Adkin, 1998)
The Gantt chart shows the time scale of each activity and their time period.
Gannt Chart Appendix 4
The cost of the item of equipment can be estimated from the purchase price of the item in previous years. This can be calculated using the Cost index knowledge. (Adkin, 1998)
Capital cost present = Cost index Present * Capital Cost Past / Cost index Past
Calculating the current price of the Fermenter 1500L.
Reference Fermenter Size and Price = 1500L and £704509
Price = £704509
1998 Cost Index = 390
2014 Cost index = 19400
Current Price of Fermenter 1500L = 606*704509/390 = £1094698
The full list of estimated delivered equipment cost of the major plant item can be found in appendix.
The total estimated equipment cost of major plant item is calculated to be £3045538.46.
Factor Principle for Capital Cost
Total plant equipment cost C = F*DCE
F = Lang Factor for DNA Vaccine Plant account for additional direct and indirect costs 8
DCE = Delivered equipment cost £3045538.46
Total capital cost C = 8 * 3045538.46 = £24364307.68
Plant costs spread over two years
C = £12182153.84
Working Capital
This is assumed to be 4% of the fixed capital cost
Scrap Value
Assumed to be 15% of the fixed capital cost.
Amortisation
Amortisation is a constant annual payment over the lifetime of the project. This is analogous to a mortgage.
Where: A= Amortisation, £, I = Initial capital cost, £, r = Interest rate n = number of years to repay the loan.
Assuming the plant life is 12years and the capitals is loaned at 5% interest.
A=12182153.84*0.051+0.05121+0.0512-1=£1374456.502
The table below show the current selling per dose and no of doses per year of the product.
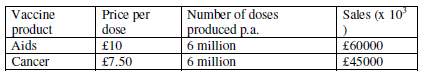
Therefore, the total income is £105million per annum, assuming this will be constant.
Major materials needed to manufacture the DNA vaccine. Obtained from catalogues. List can be found in appendix 5.
Total cost for the raw material per batch = £49851
Total per year for 282 batches = £14m
Energy Costs
To calculate the energy costs, the energy requirement of the manufacturing process needs to be reviewed. The electricity/utilities costs will be ignored. Total energy cost will be £3566.66. Calculation in appendix.
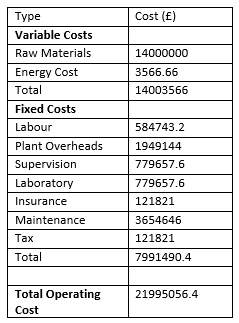
Operating Cost
All the operating Cost calculation can be found in appendix and the final answers are presented below. Calculation in appendix.
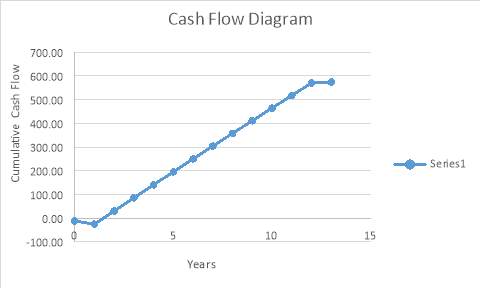
Cash Flow Diagram – This shows that the payback time is 1.5years. illustration that the project is feasible.
Return on Investment (ROI)
ROI=Cumulative Net Cash FlowInitial Investment*Plant life*100
This table shows the Return on Investment for the above project.
| Net Cash Flow (in millions) | 574.50 |
| Plant Life (in years) | 12 |
| Capital Cost (in millions) | 24.4 |
| Working Capital (in millions) | 0.976 |
| ROI as a Percentage | 188% |
The demand for DNA vaccines in large quantities at high purity for gene therapy is on the increase. Therefore, to meet the rising demand for DNA vaccines, designed a manufacturing facility to produce DNA vaccines. Applied current good manufacturing practice (cGMP) and complied with all the regulatory guidelines set up by various agencies.
The layout of the manufacturing facilities was designed in a way to allow a good waste, raw material and personnel flow to minimise the risk and contamination as all of these are an example of current good manufacturing practices which are vital for the production of the therapeutic product (DNA vaccines). Plant services, system and utilities was also designed to meet the requirement for the manufacturing facility.
To be competitive in the market, the manufacturing Plant will be constructed, commissioned and validated as fast as possible. To achieve this target, used fast track/alliance approach in creating the project schedule and carrying out dependant activates concurrently.
Finally, the costing of the DNA manufacturing plant shows that the plant is feasible and has significant profit with the payback time of only 1.5years.
Adkin, A. (1998). Design of a multi-use DNA vaccine production facility. Project report submitted for partial fulfilment for the degree of Master of Science in Biochemical Engineering., [online] 1(UCL). Available at: https://www.researchgate.net/publication/260581752_Design_of_a_multi-use_DNA_vaccine_production_facility [Accessed 5 Mar. 2017].
admin, (2014). Combined heat and power to reduce energy costs and emissions. Chem. [online] Available at: http://www.engineersjournal.ie/2014/06/05/combined-heat-and-power-to-reduce-energy-costs-and-emissions/ [Accessed 5 Mar. 2017].
AHMED, S. (2016). Advance Process Design.
Cameron, G. (2017). An Introduction to Good Manufacturing Practice (GMP) & Validation. 1st ed. Presentation Msc.
Cole, G. (1998). Pharmaceutical production facilities: Design and applications. New York: Taylor & Francis.
Cole, G., Bennett, B. and ICheme, (2003). Engineers guide to pharmaceuticals production – IChemE. Rugby: The Institution of Chemical Engineers.
Goode, D. (2017). CIP and SIP systems in Cleaning. Chemical Engineering University of Birmingham.
GSK, (2017). Gsk to produce Dna vaccines construction essay. [online] Available at: https://www.uniassignment.com/essay-samples/construction/gsk-to-produce-dna-vaccines-construction-essay.php [Accessed 5 Mar. 2017].
Healthcare, R. and Inspection, t. (2002). Rules and guidance for pharmaceutical manufacturers and distributors (the orange guide): 2014. ed. London: Pharmaceutical Press.
Hussein, S., Azmi, M., Lila, M., Yian, R., Pei, Y., Kiong, A., Ling, and Laere, E. (2016). Journal of botany. Journal of Botany, [online] 2016. Available at: https://www.hindawi.com/journals/jb/2016/4928637/ [Accessed 1 Mar. 2017].
Inc, S. (2017). Pharmaceutical production facilities – design and applications 2nd ed – graham C. Cole (Taylor & Francis, 1998).pdf | pharmaceutical drug. [online] Available at: https://www.scribd.com/doc/300136141/Pharmaceutical-Production-Facilities-Design-and-Applications-2nd-ed-Graham-C-Cole-Taylor-Francis-1998-pdf [Accessed 5 Mar. 2017].
Ingram, A. (2017). Process Control in the Pharmaceutical Industry.
Nigel, (2017). pDNA manufacture Lecture notes.
Rivière, I., Sadelain, M., Borquez-Ojeda, O., Bartido, S. and Przybylowski, M. (2017). Production of clinical-grade plasmid DNA for human phase I clinical trials and large animal clinical studies. Vaccine, [online] 25(27), pp.5013–5024. Available at: http://www.sciencedirect.com/science/article/pii/S0264410X07004768 [Accessed 1 Mar. 2017].
UK, (2015). The manufacturing of DNA vaccines engineering essay. [online] Available at: https://www.ukessays.com/essays/engineering/the-manufacturing-of-dna-vaccines-engineering-essay.php [Accessed 5 Mar. 2017].
Vilhelmsen, B. (2017). Water Systems Challenges for the Pharmaceutical Industry. MDPI Technologist: GSK.
Key
| Key for the layout of the Plant site | |
| Roads |   |
| Utilities Supply |  |
| Pipelines |  |
| Key for the layout of the Plant site | |
 |
cGMP grade Unclassified |
 |
cGMP Grade C |
 |
cGMP Grade D |
 |
Material Flow (manual Transfer) |
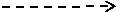 |
Material Flow (piped) |
 |
People Flow |
 |
Equipment and Waste Flow |
 |
Material and Product Flow |
| Facility Area | 504m2 |
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more