For a patient with a spinal disorder that you have treated in your clinical practice present a case study focusing on the selection of examination and management techniques.
Your assignment must include
Subjective
A 13-year-old female footballer presented with an acute aggravation of recurrent lower back pain. She reported being pushed from behind during a game three days prior, causing immediate sharp 7/10NPRS (Numerical Pain Rating Scale) pain (P1) as she went into lumbar extension, forcing her to retire from the match. The pain settled over 30 minutes to a 3/10NPRS constant ache for two days. This pattern was similar to previous aggravations; which occurred when on two instances including sprinting without a warm-up and landing heavily while horse-riding, over the prior six months (Figure 1).
Recently, a secondary pain (P2) had developed when P1 was at its worst. P2 was felt as a 4/10NRPS deep ache through the right groin and would present after two hours of sitting, easing after 20 minutes of walking. No red flags were reported. She desired a return to state-age representative level football and feared that the ongoing nature of aggravations of pain might restrict this.
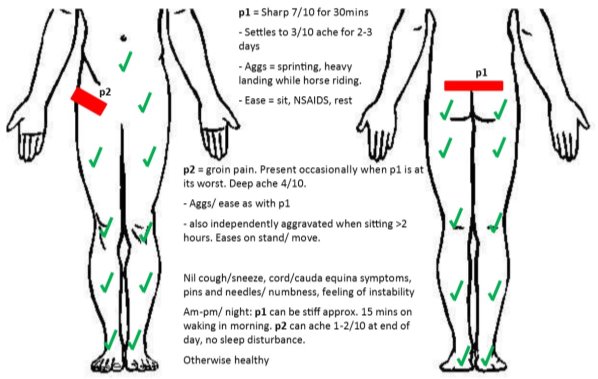 |
| Figure 1: Body chart. “p1” = primary pain, “p2” = secondary pain. |
Assessment: (* indicates key objective assessment findings used for re-assessment)
PAIVM (2, 3):
Reasoning
Rapid, ‘sharp’ pain is typically derived from A-delta nerve fibres, which are present in high density in soft-tissues (4-6). A latent, deep ‘ache’ is attributable to C-fibre activation, with Zygapophyseal capsules and joint cartilage containing a high density of C-fibres, while periosteum is also innervated by A-delta and C-fibres (4-8). Pain present at night is a distinguishing feature of bone not present in this case, thus sensation and temporal descriptions do not implicate a specific structure (9).
Unilateral groin or hip pain referral is associated with commonly implicated structures including the intervertebral disc (IVD), Sacroiliac Joint (SIJ) and Zygapophyseal facets (10, 11). Inter-facet joint space is reduced on lumbar extension and ipsilateral LF, both aggravators of the patient’s pain (11-15). IVD symptoms are more commonly associated with flexion or compression loads, while SIJ pain provocation testing was negative (2, 12).
Lumbar extension limitation is afforded by the articular processes, anterior longitudinal ligament, Psoas major and abdominal muscles (12, 16). Symptoms were not reproduced during muscle testing and spinal ligaments are highly resistant to stress (12, 16, 17). Symptoms during lumbar extension, as well as combined extension/ (R) LF, and PAIVM assessment, implicates a lumbar facet pathology (3, 12, 15, 16).
P2, temporally related with P1, may represent somatic referral, however an independent presentation during prolonged sitting implicate a separate pathology. Sustained, flexed postures (sitting), at lower intradiscal pressures, are reported to induce repetitive stress on the annulus and may predispose to annular tears (18, 19). Lateral bending plus rotation is also shown to induce high strain of annular fibres, with the highest strain at the posterolateral annulus (20).
Running involves lateral bending and contralateral rotational movements of the lumbar spine (21). The three-dimensional, whiplash-like mechanism described by the patient may disrupt the annulus of an IVD, combined with lumbar facet impaction pathology (22). A mild IVD annular tear may be aggravated by sustained sitting, while sensitisation of the Dorsal rami and Sinuvertebral nerves may explain the similarity of referred symptoms in the presence of acute pathology and inflammation (4, 6-8, 10, 12, 13).
The injury mechanism correlates with assessment findings related to P1. P2 was not aggravated during assessment. Without night pain and with a negative single leg lumbar extension test, a diagnosis for facet joint impaction with IVD Annular tear injury was made. Due to slow response to conservative management, further investigation was requested via MRI, reporting 92% sensitivity and 95% Specificity (23).
Diagnosis:
Anatomy and Biomechanics of Pars Fracture
Spondylolysis is commonly considered a fatigue fracture with genetic predisposition (24-28). Cadaver and population based skeletal studies have investigated various anatomical discrepancies correlated with spondylolysis and spondylolisthesis (14, 26, 29-35). Experimentally, fatigue failure of the neural arch most commonly occurred at the pars, with the least cycles before failure occurring at L5 (26). With increasing age IVD stiffness increases which suggests this protective stiffness is not present in adolescents, allowing load distribution posteriorly (26).
In people with spondylolysis, Pelvic Incidence (PI), a consequence of sacral and pelvic orientation (Figure 2), is outside the limits of the normal population (33, 36, 37). Two subgroups are proposed; high PI and high Sacral Slope (SS) resulting in increased lumbosacral shear stress, while low PI and low SS results in impingement of the neural arch of L5 between the L4 and Sacrum during extension (38). Facet orientation of L3-4 and L4-5 is also altered in this population, becoming increasingly coronal, thereby constraining sagittal flexion-extension motion and resulting in early joint impaction (14, 29, 37).
In typical spinal biomechanics, lumbar extension involves posterior rotation and posterior translation of the vertebral body in the sagittal plane, impacting the superior articular process on the inferior within the motion segment (12, 16, 39). Extensor muscle action increases compressive loads within the IVD, while the facet joints may bear up to 40% of this load, implicating extensor activity in this presentation (39, 40).
High PI is often observed as an anteriorly rotated pelvis and requires increased lumbar lordosis for upright posture (41, 42). Extension loading and lumbar lordosis models suggests that the highest stress in the neural arch occurs at the pars (31). In both scenarios of PI being increased or decreased, the altered facet orientation results in early joint impaction or neural arch impingement, with the combined predisposition and repetitive loads resulting in bone stress (33-38).
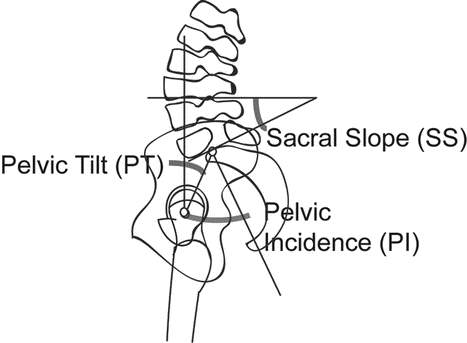 |
| Figure 2: Pelvic Incidence (PI). PI is the angle between the axis of the femoral heads to the perpendicular line from the mid-point of the sacral base. Sacral Slope the angle between the sacral base and horizontal. Pelvic Tilt is the angle between the vertical and a line intersecting the axis of the femoral head and the mid-point of the sacral base. Adapted from Roussouly et. al. 2006 (38). |
Literature addressing motor control adaptations in acute spondylolysis is lacking. A Trendelenburg gait involves poor frontal plane pelvis stability and hip adduction control during stance, creating an ipsilateral lateral flexion of the lumbar spine (43). During running, flexion-extension, lateral flexion and rotational motion of lumbar segments significantly increases above walking, with peak extension and anterior pelvic tilt occurring at toe-off with each stride cycle (21). Running induces loads of a factor of 2.7-5.7 of bodyweight with each foot-strike and disc height is lost faster than typical daily activities (44, 45).
Reduced protection from lateral flexion with a Trendelenburg gait in the presence of errant PI and a higher demand for spinal segmental control may combine to load facet joints. A forced hyperextension mechanism may have consequently further increased the posterior compressive load, leading to acute spondylolysis.
Management:
Early Management
Initial diagnosis did not consider Spondylolysis, however a pattern of posterior compression and postural/ biomechanical predisposition directed management choices towards avoidance of extension positions and motor control retraining for posterior pelvic tilting. The patient continued to suffer a 1/10(NPRS) ache over the following two weeks, prompting further investigations.
Spondylolysis diagnosis:
A lumbar brace was considered for extension restrictions and promoting reduced bone stress but was collectively rejected (46-48). Bracing does not significantly affect outcomes with 83.9% of patients reporting a positive outcome irrespective of radiological fracture healing (47). Without a fracture line or bony displacement, apposition of bone was not necessary, however reduced posterior compressive load was importantly achieved by the activity modification previously recommended (28, 33, 38, 47, 49, 50).
Bone growth occurs in response to loading, however spondylolysis is a result of supraphysiological loading and a reduced osseous adaptation capacity (26, 28, 33). Site specific muscle size is associated with increased bone mineral density and may be protective of bony lesions (51). Bone mineral density (BMD) is higher in loaded limbs of athletes in various sports, relative to impact, and maintained in osteoporotic impact trained individuals (52, 53). Improved BMD may be facilitated by addition of dynamic loading and return to sport with appropriate lumbo-pelvic mechanics, which progressively occurred on cessation of symptoms.
This patient did not enjoy early specific muscle retraining, with preference for static and dynamic motor control training, presumably due to specificity towards return to football. Spondylolysis diagnosis extended the activity restriction time-frame until symptoms clinically resolved, recommended at a minimum of six weeks, which occurred at eight weeks in this case (27, 28, 54). This prolonged restriction and prognosis was communicated, with an agreed goal of consistent running with appropriate mechanics guiding a return to sport. Motor control exercises were progressed with the goal of maintaining lumbar flexion and posterior pelvic tilt in progressively more complex movement patterns and during gait. A return to run program began at eight weeks.
Massage therapy of the Rectus Femoris and Iliopsoas was utilised, which reduces electromyographic amplitude of treated muscles, facilitating exercise induced adaptations of the antagonists; Hip Abductors/ Extensors and pelvic tilt control (55). This was preferred over stretching due to patient inability to avoid lumbar extension and a possible promotion of anterior shear of the L5 vertebra by Psoas attachment (56, 57).
Posture and motor control
Reduced anterior pelvic rotation leads to reducing lumbar lordosis and subsequently reduced facet joint compressive load (41, 42). Motor control and hip abductor/ extensor strength were addressed in exercise prescription and directed by motor learning principles with load, task complexity and antigravity requirements being progression variables (58). Improved hip abductor strength does not improve Trendelenburg gait patterns, however benefits lumbar spine pain and outcomes (43). Daily home exercises and bi-weekly physiotherapy with supervised exercise facilitated this for eight weeks, with physiotherapy reducing to weekly until week 20 (discharge).
| Exercise | Weeks | Motor control goal |
| Supine glute bridge | 1-4 | Maintain neutral pelvis rotation (sagittal plane) |
| Single leg supine glute bridge | 3-6 | |
| Kneeling squat (segment of traditional squat) | 2-6 | |
| Traditional bodyweight squat | 3-10 | |
| Single leg stance | 7-10 | Maintain neutral pelvis (frontal and sagittal plane). Maintain frontal knee alignment. |
| Traditional stationary lunge | 7-10 | |
| Forward step up | 9-12 | |
| Walking Lunges | 11-16 | |
| Single leg squat | 11-16 | |
| Single leg stance: varied stationary passing and heading ball | 11-16 | |
| Double leg squat jump | 13-16 | Maintain neutral pelvis rotation (sagittal plane). Maintain frontal knee alignment. |
| Squat jump to single leg landing | 16-20 | Maintain neutral pelvis (frontal and sagittal plane). Maintain frontal knee alignment. |
| Squat jump to double and single leg landing: heading ball | 16-20 | |
| Table 1: Overview of exercise selection with pelvic and lower limb motor control goals. Mastery of each exercise was determined by repetitions to volitional fatigue with arbitrary repetition goals based on clinical experience (i.e. 30 repetition goal for single leg squat, 120 second goal for single leg stance). | ||
Exercise selection (Table 1) was directed at muscle strength deficiencies of gluteus medius/minimus and maximus, while posterior pelvic tilt control was achievable for Meg (59). Exercises were progressed when goals for high repetitions before volitional fatigue and loss of pelvic control were met. Exercises were prescribed in line with endurance parameters (3 sets of 15-25 repetitions), while intermittent breaks were encouraged upon fatigue or failure of motor control goal, thereby facilitating development of motor control and muscular endurance (60). Late-stage exercises progressed towards complex tasks with specificity to football, the goal being return to play.
Standing posture and gait retraining was addressed throughout, due to the biomechanical predisposition to extension load to the neural arch (14, 29, 36, 38, 41, 57). Cues to avoid knee hyperextension during stance phase facilitated lumbopelvic tilt towards neutral. Twenty minutes was prescribed for daily gait practice, supported by evidence of maintained adaptations six months following 90 minutes per week for six weeks (61, 62). Gait began with focused walking and single step practice, progressing to an intermittent run program at 8 weeks, followed by continuous running at 16 weeks. Verbal feed-back on errors and successes plus therapist-assisted bio-feedback facilitated extrinsic learning, with the use of mirrors and a cognitive understanding of gait related goals facilitating a progression towards intrinsic feedback (63-65).
Outcome
The patient returned to Football training at 20 weeks and was selected for competition at 24 weeks.
Reflection
Clinical history and examination does not reliably discern spondylolysis, however spondylolysis in adolescent athletes presenting with lumbar pain occurs in 47% of cases (28, 48, 66, 67). Adolescent back pain often resolves spontaneously with modified activity; however, recurrence may suggest more sinister pathology (28, 48, 66, 67). Single leg lumbar extension testing is poorly validated for spondylolysis and the presented negative result was heavily weighted in early clinical reasoning (68, 69). Initial diagnosis did not consider these statistic, likely due to confirmation bias.
Referral for MRI investigation was delayed as per patient preference. Management aims did not adjust due to MRI results, however time-frame for activity restriction and thus return-to-play was extended beyond an initial six-week suggestion.
In future, further appreciation of adolescent propensity for bony pathology, particularly with respect to recurrence of symptoms will raise greater suspicion of pars defects and perhaps an earlier request for imaging. The present case successfully returned to sport, however this time-frame may have benefited from earlier diagnosis and thus improved compliance by the patient.
References
1. Sterling M, Brentnall D. Patient specific functional scale. Australian Journal of Physiotherapy. 2007;53(1):65.
2. Cleland J, Koppenhaver S, Su J. Netter’s Orthopaedic Clinical Examination: An Evidence-Based Approach: Elsevier Health Sciences; 2010.
3. Hengeveld E, Banks K. Maitland’s Vertebral Manipulation: Management of Neuromusculoskeletal Disorders: Elsevier Health Sciences UK; 2013.
4. Mantyh PW. The neurobiology of skeletal pain. The European journal of neuroscience. 2014;39(3):508-19.
5. Butler DS, Matheson J. The Sensitive Nervous System: Noigroup Publications; 2000.
6. Schaible HG. Peripheral and central mechanisms of pain generation. Handbook of experimental pharmacology. 2007(177):3-28.
7. Cavanaugh JM, Lu Y, Chen C, Kallakuri S. Pain generation in lumbar and cervical facet joints. The Journal of bone and joint surgery American volume. 2006;88 Suppl 2:63-7.
8. Youssef P, Loukas M, Chapman JR, Oskouian RJ, Tubbs RS. Comprehensive anatomical and immunohistochemical review of the innervation of the human spine and joints with application to an improved understanding of back pain. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2016;32(2):243-51.
9. Dimitri P, Rosen C. The central nervous system and bone metabolism: an evolving story. Calcified tissue international. 2016:1-10.
10. DePalma MJ, Director ISC, Biotech S. Multivariable analysis of the relationship between pain referral patterns and the source of chronic low back pain. Pain physician. 2012;15:171-8.
11. Fukui S, Ohseto K, Shiotani M, Ohno K, Karasawa H, Naganuma Y. Distribution of referred pain from the lumbar zygapophyseal joints and dorsal rami. The Clinical journal of pain. 1997;13(4):303-7.
12. Bogduk N. Clinical Anatomy of the Lumbar Spine and Sacrum: Elsevier/Churchill Livingstone; 2005.
13. Li G, Wang S, Passias P, Xia Q, Li G, Wood K. Segmental in vivo vertebral motion during functional human lumbar spine activities. European Spine Journal. 2009;18(7):1013-21.
14. Masharawi YM, Alperovitch-Najenson D, Steinberg N, Dar G, Peleg S, Rothschild B, et al. Lumbar facet orientation in spondylolysis: a skeletal study. Spine. 2007;32(6):E176-E80.
15. Cavanaugh JM, Ozaktay AC, Yamashita HT, King AI. Lumbar facet pain: biomechanics, neuroanatomy and neurophysiology. J Biomech. 1996;29(9):1117-29.
16. Izzo R, Guarnieri G, Guglielmi G, Muto M. Biomechanics of the spine. Part I: spinal stability. European journal of radiology. 2013;82(1):118-26.
17. Panjabi MM, GOEL VK, TAKATA K. Physiologic Strains in the Lumbar Spinal Ligaments: An In VitroBiomechanical Study. Spine. 1982;7(3):192-203.
18. Wilke HJ, Neef P, Caimi M, Hoogland T, Claes LE. New in vivo measurements of pressures in the intervertebral disc in daily life. Spine. 1999;24(8):755-62.
19. Costi JJ, Stokes IA, Gardner-Morse M, Laible J, Scoffone HM, Iatridis J. Direct measurement of intervertebral disc maximum shear strain in six degrees of freedom: motions that place disc tissue at risk of injury. Journal of biomechanics. 2007;40(11):2457-66.
20. Schmidt H, Kettler A, Heuer F, Simon U, Claes L, Wilke H-J. Intradiscal pressure, shear strain, and fiber strain in the intervertebral disc under combined loading. Spine. 2007;32(7):748-55.
21. Schache AG, Blanch P, Rath D, Wrigley T, Bennell K. Three-dimensional angular kinematics of the lumbar spine and pelvis during running. Human Movement Science. 2002;21(2):273-93.
22. Ivancic PC. Mechanisms and mitigation of head and spinal injuries due to motor vehicle crashes. journal of orthopaedic & sports physical therapy. 2016;46(10):826-33.
23. Rush JK, Astur N, Scott S, Kelly DM, Sawyer JR, Warner Jr WC. Use of magnetic resonance imaging in the evaluation of spondylolysis. Journal of Pediatric Orthopaedics. 2015;35(3):271-5.
24. Beutler WJ, Fredrickson BE, Murtland A, Sweeney CA, Grant WD, Baker D. The natural history of spondylolysis and spondylolisthesis: 45-year follow-up evaluation. Spine (Phila Pa 1976). 2003;28(10):1027-35; discussion 35.
25. Cavalier R, Herman MJ, Cheung EV, Pizzutillo PD. Spondylolysis and spondylolisthesis in children and adolescents: I. Diagnosis, natural history, and nonsurgical management. Journal of the American Academy of Orthopaedic Surgeons. 2006;14(7):417-24.
26. Cyron BM, Hutton WC. The fatigue strength of the lumbar neural arch in spondylolysis. The Journal of bone and joint surgery British volume. 1978;60-b(2):234-8.
27. Haun DW, Kettner NW. Spondylolysis and spondylolisthesis: a narrative review of etiology, diagnosis, and conservative management. Journal of Chiropractic Medicine. 2005;4(4):206-17.
28. Syrmou E, Tsitsopoulos PP, Marinopoulos D, Tsonidis C, Anagnostopoulos I, Tsitsopoulos PD. Spondylolysis: A review and reappraisal. Hippokratia. 2010;14(1):17-21.
29. Don AS, Robertson PA. Facet joint orientation in spondylolysis and isthmic spondylolisthesis. Clinical Spine Surgery. 2008;21(2):112-5.
30. Foreman P, Griessenauer CJ, Watanabe K, Conklin M, Shoja MM, Rozzelle CJ, et al. L5 spondylolysis/spondylolisthesis: a comprehensive review with an anatomic focus. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2013;29(2):209-16.
31. Terai T, Sairyo K, Goel V, Ebraheim N, Biyani A, Faizan A, et al. Spondylolysis originates in the ventral aspect of the pars interarticularis. Bone & Joint Journal. 2010;92(8):1123-7.
32. Ward CV, Latimer B, Alander DH, Parker J, Ronan JA, Holden AD, et al. Radiographic assessment of lumbar facet distance spacing and spondylolysis. Spine. 2007;32(2):E85-E8.
33. Whitesides TE, Jr., Horton WC, Hutton WC, Hodges L. Spondylolytic spondylolisthesis: a study of pelvic and lumbosacral parameters of possible etiologic effect in two genetically and geographically distinct groups with high occurrence. Spine (Phila Pa 1976). 2005;30(6 Suppl):S12-21.
34. Yao Q, Wang S, Shin J-H, Li G, Wood KB. Lumbar facet joint motion in patients with degenerative spondylolisthesis. Journal of spinal disorders & techniques. 2013;26(1):E19.
35. Zehnder SW, Ward CV, Crow AJ, Alander D, Latimer B. Radiographic assessment of lumbar facet distance spacing and pediatric spondylolysis. Spine. 2009;34(3):285-90.
36. Peleg S, Dar G, Steinberg N, Masharawi Y, Been E, Abbas J, et al. Sacral orientation and spondylolysis. Spine. 2009;34(25):E906-E10.
37. Weinberg DS, Xie KK, Liu RW, Gebhart JJ, Gordon ZL. Increased Pelvic Incidence is Associated with a More Coronal Facet Orientation in the Lower Lumbar Spine: A Cadaveric Study of 599 Lumbar Spines. Spine. 2016.
38. Roussouly P, Gollogly S, Berthonnaud E, Labelle H, Weidenbaum M. Sagittal alignment of the spine and pelvis in the presence of L5–S1 isthmic lysis and low-grade spondylolisthesis. Spine. 2006;31(21):2484-90.
39. El-Bohy AA, Yang K-H, King AI. Experimental verification of facet load transmission by direct measurement of facet lamina contact pressure. Journal of biomechanics. 1989;22(8):931-41.
40. ÖRTENGREN R, ANDERSSON GB, Nachemson AL. Studies of relationships between lumbar disc pressure, myoelectric back muscle activity, and intra-abdominal (intragastric) pressure. Spine. 1981;6(1):98-103.
41. Labelle H, Mac-Thiong J-M. Role of the Pelvis in the Diagnosis and Management of L5-S1 Spondylolisthesis. Spondylolisthesis: Springer; 2015. p. 107-18.
42. Legaye J, Duval-Beaupere G, Hecquet J, Marty C. Pelvic incidence: a fundamental pelvic parameter for three-dimensional regulation of spinal sagittal curves. European Spine Journal. 1998;7(2):99-103.
43. Kendall K, Schmidt C, Ferber R. The relationship between hip-abductor strength and the magnitude of pelvic drop in patients with low back pain. Journal of Sport Rehabilitation. 2010;19(4):422-35.
44. Cappozzo A, Berme N. Loads on the lumbar spine during running. Biomechanics IX-B. 1985:97-100.
45. White TL, Malone TR. Effects of running on intervertebral disc height. Journal of Orthopaedic & Sports Physical Therapy. 1990;12(4):139-46.
46. Garet M, Reiman MP, Mathers J, Sylvain J. Nonoperative Treatment in Lumbar Spondylolysis and Spondylolisthesis: A Systematic Review. Sports Health. 2013;5(3):225-32.
47. Klein G, Mehlman CT, McCarty M. Nonoperative treatment of spondylolysis and grade I spondylolisthesis in children and young adults: a meta-analysis of observational studies. Journal of Pediatric Orthopaedics. 2009;29(2):146-56.
48. McCleary MD, Congeni JA. Current concepts in the diagnosis and treatment of spondylolysis in young athletes. Curr Sports Med Rep. 2007;6(1):62-6.
49. Bouras T, Korovessis P. Management of spondylolysis and low-grade spondylolisthesis in fine athletes. A comprehensive review. European Journal of Orthopaedic Surgery & Traumatology. 2015;25(1):167-75.
50. Dietrich M, Kurowski P. The importance of mechanical factors in the etiology of spondylolysis. A model analysis of loads and stresses in human lumbar spine. Spine (Phila Pa 1976). 1985;10(6):532-42.
51. Abe T, Loenneke J, Young K, Nahar V, Hollaway K, Stover C, et al. Site-specific associations of muscle thickness with bone mineral density in middle-aged and older men and women. Physiology International (Acta Physiologica Hungarica). 2016;103(2):202-10.
52. Fehling P, Alekel L, Clasey J, Rector A, Stillman R. A comparison of bone mineral densities among female athletes in impact loading and active loading sports. Bone. 1995;17(3):205-10.
53. Bolam KA, Skinner TL, Jenkins DG, Galvao DA, Taaffe DR. The Osteogenic Effect of Impact-Loading and Resistance Exercise on Bone Mineral Density in Middle-Aged and Older Men: A Pilot Study. Gerontology. 2015;62(1):22-32.
54. Iwamoto J, Sato Y, Takeda T, Matsumoto H. Return to sports activity by athletes after treatment of spondylolysis. World J Orthop. 2010;1(1):26-30.
55. Sefton JM, Yarar C, Carpenter DM, Berry JW. Physiological and clinical changes after therapeutic massage of the neck and shoulders. Manual therapy. 2011;16(5):487-94.
56. Nourbakhsh MR, Arabloo AM, Salavati M. The relationship between pelvic cross syndrome and chronic low back pain. Journal of Back and Musculoskeletal Rehabilitation. 2006;19(4):119-28.
57. Pel J, Spoor C, Pool-Goudzwaard A, van Dijke GH, Snijders C. Biomechanical analysis of reducing sacroiliac joint shear load by optimization of pelvic muscle and ligament forces. Annals of biomedical engineering. 2008;36(3):415-24.
58. Sairyo K, Sakai T, Yasui N, Dezawa A. Conservative treatment for pediatric lumbar spondylolysis to achieve bone healing using a hard brace: what type and how long? Clinical article. Journal of Neurosurgery: Spine. 2012;16(6):610-4.
59. Reiman MP, Bolgla LA, Loudon JK. A literature review of studies evaluating gluteus maximus and gluteus medius activation during rehabilitation exercises. Physiotherapy theory and practice. 2012;28(4):257-68.
60. Medicine ACoS. ACSM’s guidelines for exercise testing and prescription: Lippincott Williams & Wilkins; 2013.
61. Shull PB, Silder A, Shultz R, Dragoo JL, Besier TF, Delp SL, et al. Six-week gait retraining program reduces knee adduction moment, reduces pain, and improves function for individuals with medial compartment knee osteoarthritis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31(7):1020-5.
62. Teran-Yengle P, Cole KJ, Yack HJ. Short and long-term effects of gait retraining using real-time biofeedback to reduce knee hyperextension pattern in young women. Gait & Posture. 2016.
63. Winstein CJ. Knowledge of results and motor learning—implications for physical therapy. Physical therapy. 1991;71(2):140-9.
64. Magill RA, Anderson D. Motor learning and control: Concepts and applications: McGraw-Hill New York; 2007.
65. Gentile A. Movement science: Implicit and explicit processes during acquisition of functional skills. Scandinavian journal of occupational therapy. 1998;5(1):7-16.
66. Leone A, Cianfoni A, Cerase A, Magarelli N, Bonomo L. Lumbar spondylolysis: a review. Skeletal radiology. 2011;40(6):683-700.
67. Micheli LJ, Wood R. Back pain in young athletes. Significant differences from adults in causes and patterns. Archives of pediatrics & adolescent medicine. 1995;149(1):15-8.
68. Grødahl LHJ, Fawcett L, Nazareth M, Smith R, Spencer S, Heneghan N, et al. Diagnostic utility of patient history and physical examination data to detect spondylolysis and spondylolisthesis in athletes with low back pain: A systematic review. Manual Therapy. 2016;24:7-17.
69. Masci L, Pike J, Malara F, Phillips B, Bennell K, Brukner P. Use of the one‐legged hyperextension test and magnetic resonance imaging in the diagnosis of active spondylolysis. Br J Sports Med. 2006;40(11):940-6.
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more