
Xanthines are purine alkaloids synthesised in plants such as coffee, tea, and cacao, with the most known xanthine being caffeine, theobromine and theophylline. Their production is limited in the aforementioned plant species compared to other plant alkaloids such as morphine, nicotine and strychnine, which are widely expressed across the plant kingdom. Caffeine is the world’s most consumed psychoactive drug, with approximately 74% of it used as beverages, 25% utilised for pharmaceutical formulations and the remainder for other purposes.3 It is chemically known as 1,3,7-trimethylxanthine, with a complex purine-based structure, chemically associated to adenine and guanine found in DNA and RNA
 Caffeine’s also found in human tissues as well as various organisms. It is appreciated for its role in stimulating the CNS, kidneys, bronchial smooth muscle and relaxing the cardiac muscle.2,3 As a therapeutic, caffeine is administered as a powdered form, but the psychoactive substance found in tea leaves constitutes 2-5% caffeine per weight. Caffeine is used to treat apnea of prematurity, fatigue, in analgesic preparations with aspirin and paracetamol, and in various other roles including night duties (promoting alerting effect by antagonising adenosine A1 and A2A receptors in the brain), and in sports as a performance-enhancer.
Caffeine’s also found in human tissues as well as various organisms. It is appreciated for its role in stimulating the CNS, kidneys, bronchial smooth muscle and relaxing the cardiac muscle.2,3 As a therapeutic, caffeine is administered as a powdered form, but the psychoactive substance found in tea leaves constitutes 2-5% caffeine per weight. Caffeine is used to treat apnea of prematurity, fatigue, in analgesic preparations with aspirin and paracetamol, and in various other roles including night duties (promoting alerting effect by antagonising adenosine A1 and A2A receptors in the brain), and in sports as a performance-enhancer.
Because of the prerequisites for its usage in beverages and its significant potential in the pharmaceutical industry, caffeine has been isolated primarily from coffee. However, the increasing demand for naturally derived caffeine for consumption necessitates additional other sources, for its extraction.4
Contrastive to its utilisation and because it is unregulated, there has been increasing concerns pertaining its connection to adverse side-effects on human physiology regarding its high consumptions by some, which is linked with tachycardia, arrhythmia, muscle tremors, headache, coma, or fatality.4,2,3 As such, alternative forms of beverages have been decaffeinated (e.g., green tea) to eliminate the described potential side-effects and the demand for alternative forms of beverages goes hand in hand with increasing environmental-friendly techniques used to decaffeinate the crude product.
This article, therefore, looks at the water extracting method for the isolation of methylxanthines and using thin-layer-chromatography and IR spectroscopy to characterise individual methylxanthines, as well as discussing their Rf values, the use of caffeine in cold and flu remedies, and a brief look into the caffeine market.
Preparation of Tea solution
200ml of water was placed into a beaker and the solution was allowed to boil using a Bunsen burner. Tea (10.3052g) was then added into the solution and boiled for approximately 15 minutes. The beaker was then removed from the heat and 50ml sulphuric acid was then added to the hot solution and shake to ensure no emulsion occurs. The solution was later allowed to cool at room temperature and then filtered using Buchner system.
Extraction of Caffeine
20ml of 10% sulphuric acid was added to the filtrate and stirred. Sulphuric acid converts the tannins to their salts, therefore, making them insoluble in chloroform, though soluble in water. The solution was then extracted by 3 successive washes of 50ml chloroform using a separating funnel, collecting the organic layer for each consecutive wash. The mixture of the solution was frequently shaken with occasional venting to prevent pressure buildup. The extracts were collected and dried by adding the catalyst anhydrous sodium sulphate for 10-15mins, removing all the water, leaving behind a fine powder.
Isolation of The Caffeine
Remove the sodium sulphate by filtration using the Buchner system. The chloroform was then evaporated by the use of a rotary evaporator, leaving behind the methylxanthine crystals. The weight of caffeine was then measured and the yield calculated.
Thin Layer Chromatography
TLC was conducted at room temperature and was used to verify the presence of caffeine by obtaining the methylxanthines, which was re-dissolved in 2ml of chloroform, 1ml of which was examined under TLC along with reference solutions and mother liquor. The UV-absorbing methylxanthines absorb UV light and so fluoresce agent (dichloromethane) in the stationary phase was used for visualisation in the UV254nm.
On the TLC plate (Silica gel GF254; Dimension: 5cm x 20cm), a 1cm line was drawn above the base of one end of the plate. Capillary micropipette to place a spot of the reference solution (Caffeine, theobromine, theophylline, and mother liquor) along the line drawn on the plate and labelled as appropriate to reduce confusion with the other samples, which were also labelled as appropriate. This step was repeated using different micropipettes for each sample, creating spots at about 1cm from each other. The TLC plate was then placed upright in the TLC chamber contacting the developing solvent (Chloroform: Acetone: N-Butanol: 30% Ammonia, at 30:30:40:10 ratio) at a level below 0.5cm (the origin) and sealed with using a watch glass. The solvent was allowed to migrate along the TLC plate so it reaches at least 1cm from the top and once the solvent had evaporated, the plate subsequently visualised under UV light as most organic compounds are colourless in the naked eye, facilitated by the fact that the TLC plate contains chemical additives that fluorescent under UV-light.
The remaining methylxanthine solution of caffeine obtained was used to produce an IR spectrum to deduce the functional groups in the compound. The measured spectral range was between 600 – 4000 cm . The sample was placed on a sampling window and spectral data collected using a spectrum software (PerkinElmer Spectrum Express version 1.02.00, UK).10
. The sample was placed on a sampling window and spectral data collected using a spectrum software (PerkinElmer Spectrum Express version 1.02.00, UK).10
Table 1: Weight of crude caffeine
|
Caffeine |
0.1606g |
Percentage yield = Mass of crude caffeine / mass of tea bags x 100%
= 0.1606g / 10g Ã- 100%
= 1.606%
Table 2: Distance travelled by the standard solutions and Methylxanthines (mm)
|
C |
TB |
TP |
ML |
Sample |
|
36 |
24 |
18 |
Spot 1= 34 Spot 2= 26 Spot 3= 16 |
34 |
Note: C=caffeine, TB = Theobromine, TP = Theophylline, ML = Mother Liquor, S = Sample
Rf value= Distance travelled by the compound (mm)
Distance travelled by the solvent (mm)
Caffeine = 36mm/38mm = 0.95
Theobromine =24mm/38mm = 0.63
Theophylline =18mm/38mm = 0.47
Mother Liquor spot 1= 34mm/38mm = 0.89
ML spot 2 = 26mm/38mm = 0.68
ML spot 3 = 15mm/38mm = 0.39
S= 34mm/38mm = 0.89
Table 3: Rf values of the standard solutions and Methylxanthines (mm)
|
C |
TB |
TP |
ML |
Sample |
|
0.95 |
0.63 |
0.47 |
Spot 1= 0.89 Spot 2= 0.68 Spot 3= 0.42 |
0.89 |
Note: C=caffeine, TB = Theobromine, TP = Theophylline, ML = Mother Liquor, S = Sample
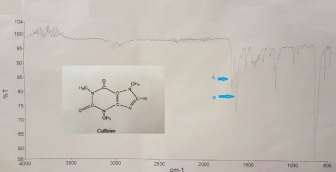
Figure 1: IR spectra of the caffeine. Peak 3000 cm-1 represents amides and amines, and peaks between the regions 1700 -1600cm-1 indicates the presence of alkene in caffeine. A and B shows the carboxyl groups – C=O bands of carbon-2 and 6 in region 1700-1659 cm-1.
Figure 2: IR spectrum of the standard. The peak around 3000 is due to amides and amines. Peaks 1700-1600 is alkene in the caffeine molecule
The structure of caffeine (Figure 3) is a function of how it behaves and interacts with other molecules and defines its properties such as solubility (e.g., due to the presence of nitrogen atoms), boiling point, as well as the melting point. The tertiary purine-based caffeine constitutes an amine, amide and alkene function group, all containing lone pairs of electrons on the nitrogen atom. The achiral molecule is polar in nature due to the electronegativity difference between carbon-oxygen and carbon-nitrogen covalent bonds due to dipole-dipole interactions, London dispersion forces, and hydrogen bonding once in water. The higher melting point of this molecule is as a result of these strong intermolecular forces and would necessitate high energy to break the associated bonds.1,2
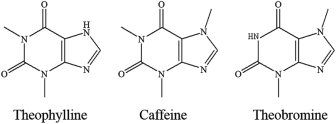
Figure 3: Structures of caffeine, theophylline and theobromine
Figure 3 indicates that the methylxanthines share similar structures (purine ring), but the slight molecular difference in structure results in the differences in properties. The difference in structure is based on the positioning of the methyl groups. Caffeine has three methyl group in carbon-1, 3 and 7; theobromine has two methyl groups on carbon-3 and 7;
and theophylline has two methyl group on carbon-1 and 3, and is deficit of methyl group at position 7 and so has only a proton that can be donated, the same for theobromine having a proton on carbon-1, making them a weakly amphoteric compared to caffeine (a base compound).2
The TLC result shows the distance travelled by caffeine, theobromine and theophylline were 0.95, 0.63 and 0.47mm, respectively (Table 2). This implies that caffeine is more of a solvent compared to theobromine and theophylline. Mother Liquor contains three substances as shown by spot 1 to spot 3 in Table 3, with spot 1, 2 and 3 having Rf values of 0.89, 0.68 and 0.42, respectively. This means that spot 1 and caffeine are more attracted to the mobile phase, interacting less with the polar adsorbent (that’s interaction with the silanol group of the silica gel, with the most prevailing interactive force being dipole-dipole) as the compound is less polar, restricted to form hydrogen bonds by its three methyl groups (these contain electrophilic sites and the compound possess electrophilic and nucleophilic function groups, but caffeine is not a proton donor so less polar to adsorb) and so having large Rf values, consequently spent less time travelling towards the solvent front as it is more soluble in the mobile phase. Theoretically, compounds that are less polar characteristically dissolve in the solvent, thus migrate faster, and that the silanol group of the silica surface is highly polarised and is capable of forming dipole-dipole and hydrogen bonds. The more polar compounds such as theophylline (spot 3) strongly binds to the silanol group of the adsorbent or the stationary phase as they’re both capable of hydrogen bonding (theophylline is more of a proton donor thus has more electronegative substituents than for example, caffeine, so binds tightly to the stationary phase), and so moved slower than both spot 1 and 2, but also spends more time closer the origin, held by the resistive force of the sorbent.5,28 Spot 1 is likely to be containing samples of caffeine as they travel almost the same distance. Spot 2 is likely to contain samples of theobromine and spot 3 contains samples of theophylline as they have almost similar Rf values. IR spectroscopy was employed to elucidate the types of the functional groups in an unknown sample. The results from the IR (Figure 1) shows the presence of a compound indicated by the energy speaks. The spectrometer produced a graph based on the measurements of the photon within 600 – 4000 cm-1 frequencies. Comparison between the IR spectrum of the standard solution (Figure 2) and that of Figure 1 confirms the likeliness in functional groups shared between these solutions. Figure 1 indicates photon energy peak visible at 3000 cm-1, representing amides and aliphatic amines6, and peaks between the regions 1700 -1600cm-1 are due to the presence of alkene in caffeine molecule9. A and B specifies the carboxyl groups (C=O) of carbon-2 and carbond-6 in region 1700-1659
cm-1, indicating the most intense bands.17
Opinion on the use of caffeine in cold and flu remedies
The effects of caffeine vary around the body and are dependent on the dose limit (400mg), at which beyond this parameter will elicit a range of physiological effects including muscle tremors, stomach upset, urinary incompetence, and a possible death. Below this dose limit, however, its effect is less detrimental to health.11 Due to it being readily available in foodstuffs and medicines, some may not be aware of the imposing dangers of caffeine, coupled with the fact that the FDA and the European guidelines consider caffeine not being a nutrient, but a natural ingredient found in beverages and so does not require identification in labelling of caffeinated product unless there’s added caffeine in the product.12 This makes it a daunting task for those tracking their caffeine intake especially those that are more vulnerable to its side-effects.
Caffeine is issued both as a prescription and as an OTC medication treating various conditions from lethargy to being used as an adjuvant in analgesic, as well as in flu or cold remedies.12 Flu causes rhinorrhea, resulting in loss of fluid, which is counter to sustaining the body’s fluid balance needed for healthy wellbeing. The elderly are the most at risk if not hydrated and the problem exacerbates with the consumption of diuretic substances including any of the methylxanthines.14 A literature review by R. J. Maughan and colleague of caffeine ingestion and its effects on fluid balance assessed various age groups (adults) and the elderly of both sexes. Robertson et al. (1978) reported that R. J. Maughan and colleague administered a single dose of caffeine (250mg) and a placebo to the subjects and urine was accumulated for 3hrs. The result produced an ‘increase in urine output from 366 ± 30 mL (mean ± SD) on the placebo trial to 469 ± 43 mL on the caffeine trial, accompanied by an increase in urinary sodium excretion‘. However, in the same report by Robertson et al., other studies indicated that the diuretic effect of small doses of caffeine had minimal effects, which may be in the same dose range in these flue/cold remedies. It was also reported that long-term caffeine users are not susceptible to this diuretic effect and may not lose water via urine output14, but those that are may be disposed to electrolyte abnormalities (e.g., natriuresis) to kidney dysfunction. The mechanism in which caffeine induces diuresis is not yet clear, but it is believed that the compound acts as a phosphodiesterases inhibitor in the kidneys, along with its antagonistic effect on adenosine receptors.15 Another complication that may arise using caffeine remedies is the possibility of drug-drug interactions such as in the case of taking tizanidine
(muscle relaxant), causing low blood pressure and dizziness16, or its inhibitory effect on the antipsychotic medications clozapine and olanzapine, metabolised by CYP1A2. Caffeine may also pose as a competitive inhibitor of CYP1A2 if metabolised at a slower rate compared to an administered drug, thus minimising the drug’s plasma concentration with the likelihood of toxicity.20
The compounding benefits of caffeine, when consumed within physiological limits, cannot be contested. Studies have shown that the groups most at risk of caffeine overdose are young people/children and adolescents due to the lack of awareness and incorrect social perception regarding the benefits versus harmful effects.17 It was reported by the American National Poison Data System that 6,309 cases related to caffeine overdose. A recent article by the telegraph newspaper reported that some students came close to fatality upon accidentally overdosing on caffeine (consumed 30000mg) and were placed on dialysis to remove the intoxication from the kidneys.19 So, in support of it still being a legal stimulant, not only does the benefits outweighs the adverse effects, the compound’s plasma half-life is approximately 5hrs. This fast pharmacokinetics or elimination via urinary excretion entails that its concentration in the blood will always be regulated, adverse side-effects occurring or at least decrease its effects.20
Due to caffeine being readily available, there are no age limits for their purchase and are not costly either as beverages. The FDA and EMA must assume responsibilities in engaging and communicating with the most at-risk groups, using public education campaign, and firmly addressing the potential risks of overdosing, especially when using multiple caffeine products in combination, and also labelling of such products, indicating the caffeine concentration, as well as targeting the medium used by these at-risk groups such as social media, the internet, and television. The European legislation, however, has taken the incentive in labelling beverages containing caffeine equal to or over 150mg caffeine per litre, affirming in their statement, “High caffeine content. Not recommended for children or pregnant or breastfeeding women“.17 Additionally, self-monitoring of caffeine concentration by providing device similar to those used by diabetic patients may assist with staying within physiological limits, but also restricting accessibility (age-dependent) might just be one way to control the likelihood of abuse.
As a commodity, caffeine can be obtained in various forms for many applications (cosmetics, medical, etc.) and there is always a huge demand,
which are popular amongst young people, particularly with the emergence of caffeine-fuelled energy drinks that are used to mix alcohols in social venues.21 Compared with other drugs, the Global Drug Survey 2014 (Figure 4) reported that caffeinated energy drinks were the fourth most drug bought (45.9% prevalence use) after alcohol, tobacco, and cannabis.
This illustrates caffeine’s importance both at physiological level, and as a big earner for the industry’s major players in global caffeine market (Pfizer, Boehringer Ingelheim, CSPC Pharma, BASF, and Cocam) as it is consumed by 90% of the world’s population.24,25 One of the drivers of the industry is the production of coffee, which is forecasted to produce 156.6 million bags in 2016/17, and global consumption to be 153.3 million bags.26 This points out the growing demand for caffeinated products, predominantly in traditional markets including Canada, EU, USA, Japan, Norway and Switzerland, but also in emerging markets; Turkey, Algeria, Russia.27
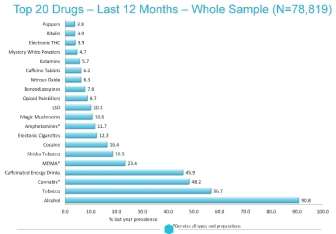
Figure 4: Prevalence of top 20 drug use. Caffeinated drinks was among the most drugs bought in the year 2014.21
It is concluded by the study that the amount of caffeine extracted in tea was almost consistent with the theoretical constituents per weight, 2-5%. This research yields 0.1606g (1.606% ) from 10kg of tea bag and so was impossible to recover 100% of caffeine, greatly impacted by the fact that the reaction was never at completion, not all the caffeine was extracted through the funnel separation, loss of product may have occurred due to emulsions, discrepancies with the instruments due to factors affecting calibration, and steaming during brewing affects the mass of the extracted caffeine. One way to improve the percentage yield may be to explore different organic solvents.
Although caffeine has numerous health benefits within physiological optima, it is also detrimental and causes death if these limits are breached. As such, healthcare authorities, as well as the caffeine industry must put in place measures so it is better regulated, and may mean being transparent about the health benefits/risk factors, and spread this awareness in all media used by their target users, especially young people as this is the group that are less aware of the risks.
References
 Caffeine. Technology, Products, Market, Manufacturing. [2017 Feb 10]. http://www.primaryinfo.com/industry/caffeine.htm
Caffeine. Technology, Products, Market, Manufacturing. [2017 Feb 10]. http://www.primaryinfo.com/industry/caffeine.htmYou have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more