Introduction
In this practical, an immunosorbent assay was performed to determine the outcome of a Synacthen test. This test is used to assess adrenal gland functions. It is routinely used to diagnose adrenal insufficiency and other related conditions. It works by giving a patient a dose of synthetic adrenocorticotropic hormone (ACTH) (Synacthen) in the morning after taking a baseline serum sample. The Synacthen will normally stimulate the adrenal glands to produce cortisol. Further serum sample are taken post dose (after 30 and 60 minutes) to determine if there is an expected increase in cortisol levels. If there is no response from the adrenal glands (low levels of cortisol), the patient is then diagnosed with adrenal insufficiency (Ref).
The levels of cortisol are measured by using an enzyme linked immunosorbent assay (ELISA). This method uses specific monoclonal antibodies that target cortisol. These antibodies are coated to the plate, once the sample is in contact with the antibodies, the cortisol present in the sample will bind to the specific coated antibodies to form an antibody-antigen complex (Ab-Ag complex). This complex is then bound to a secondary detection antibody which is coupled to horseradish peroxidase (HRP). This secondary antibody creates a coloured signal in the presence of TMB substrate due to the HRP. The reaction is then stopped with a stop solution and the coloured signal can then be detected by a spectrophotometer. The intensity of the signal is correlated to the concentration of Ab-Ag complexes.
Materials
96 well plate
Pipette and tips
Primary antibody
Patient serum sample x6 (2 samples per patient)
Secondary antibody
Wash buffer
TMB substrate
Hydrochloric acid HCl (stop solution)
Plate reader
Method
Firstly, a first washing step was performed on the pre-coated plate. This was done by adding 200µl of wash buffer to each well of the provided pre-coated plate. The plate was then emptied and the gently tapped on a cloth until all the buffer was removed. This was performed 3 times in a row.
Then, 100µl of the samples were added to a separate well in duplicate and the plate was left to incubate at room temperature for 30 minutes.
After the incubation at room temperature, a second washing step was performed.
100µl of the secondary antibody was then added to each well and the plate was left to incubate for 60 minutes at room temperature.
After incubation, a third washing step was performed.
100µl of TMB substrate was added to each well and the reaction was left to develop for 15 minutes.
Finally, 100µl of stop solution (HCl) was added to each well to stop the reaction and the plate was read at 450nm on the plate reader.
Results
The results below were provided to interpret the cortisol levels of the 3 patients:
Table 1. Provided results
Standards:
|
[Cortisol] nM |
Absorbance |
|
0 |
0.046 |
|
50 |
0.060 |
|
200 |
0.132 |
|
500 |
0.200 |
|
750 |
0.339 |
|
1000 |
0.482 |
Samples and internal quality controls:
|
Sample |
Absorbance |
|
IQC1 |
0.052 |
|
IQC2 |
0.161 |
|
IQC3 |
0.241 |
|
Patient 1 Sample 1 |
0.081 |
|
Patient 1 Sample 2 |
0.263 |
|
Patient 2 Sample 1 |
0.069 |
|
Patient 2 sample 2 |
0.138 |
|
Patient 3 sample 1 |
0.050 |
|
Patient 3 sample 2 |
0.049 |
By using the results provided from the standards, a standard curve can be plotted.
Figure 1. Standard curve of the absorbance over the concentration
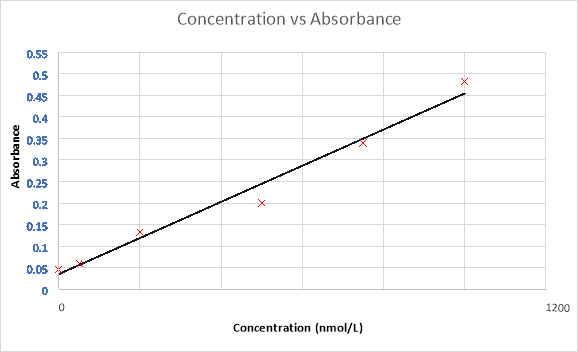
Then, by extrapolating the known absorbance from the QCs and samples, corresponding concentrations can be determined as shown below.
Table 3. Quality control results
|
QC (45 nmol/L) |
QC (315 nmol/L) |
QC (520 nmol/L) |
|
|
absorbance |
0.052 |
0.161 |
0.241 |
|
concentration |
48.47 |
302.75 |
489.37 |
|
Bias % |
7.71785044 |
3.889442 |
5.889947428 |
The accuracy of the controls is calculated as absolute bias (% RE, where relative error [RE] = [measured value – actual value]/ actual value). The bias is within the 20% range for every quality control meaning that the assay is accurate and that the results generated from the assay are validated (De silva EMEA).
Finally, by extrapolating the provided absorbance of the samples on the standard curve, a corresponding concentration can be determined.
Table 4. Sample results
|
sample 1 (t=1) |
sample 1 (t=2) |
sample 2 (t=1) |
sample 2 (t=2) |
sample 3 (t=1) |
sample 3 (t=2) |
|
|
Absorbance |
0.081 |
0.263 |
0.069 |
0.138 |
0.05 |
0.049 |
|
Concentration (nmol/L) |
116.12 |
540.69 |
88.13 |
249.09 |
43.81 |
41.47 |
Discussion
As described earlier, assays measuring cortisol levels in the blood are used to interpret Synacthen tests. In this case, 3 patients had undergone a synacthen test, a baseline serum sample and a second serum sample taken after 30 minutes post synacthen dose were taken. The samples were analysed and the cortisol levels were determined for each sample. Using the results obtained from the cortisol assay, a clinical interpretation can be done.
According to guidelines, adrenal insufficiency is ruled out if the basal cortisol level is greater than 180 nmol/L, if the increase of cortisol levels 30 minutes post dose is greater than 200nmol/L or if the maximum serum cortisol level is greater than 500-600nmol/L (reference ranges vary depending on the laboratory) (https://www.nbt.nhs.uk/sites/default/files/Short%20Synacthen%20Test.pdf)( http://www.pathology.leedsth.nhs.uk/dnn_bilm/Investigationprotocols/Synacthentestsshortlong/StandardShortSynacthenTest.aspx)( http://www.webmd.com/a-to-z-guides/cortisol-14668#2) (https://cks.nice.org.uk/addisons-disease#!diagnosisadditional).
Patient 1 is a 65 year old male which performed a synacthen test after a surgery to remove a pituitary tumour to assess the adrenal functions. The baseline cortisol levels at time 0 minutes was 116.2nmol/L and the cortisol levels after 30 minutes post dose was 540.69nmol/L. Following the guidelines, this patient does not suffer from adrenal insufficiency even though his basal cortisol levels are lower than 180nmol/L. This low basal level can be explained because part of the pituitary was removed due to a tumour, meaning that the ACTH signal from the pituitary gland to the adrenal cortex will be diminished, therefore the basal cortisol level is decreased. But since the cortisol levels at 30 minutes have increased by more than 200nmol/L and are above 500nmol/L, it can be determined that the Synacthen response is normal.
Patient 2 is an asthmatic 15 year old female that performed a synacthen test following a long term steroid treatment. Her basal cortisol level measured was 88.13nmol/L followed by a 30 minute cortisol level of 249nmol/L. Following the guidelines, it can be said that this patient suffers from adrenal insufficiency. This diagnostic is given since the increase in cortisol levels after 30 minutes post synacthen dose is below 200nmol/L, furthermore, the cortisol levels at 30 minutes is greatly below 500nmol/L.
The 3rd and final patient is a 38 year old male, admitted to A&E after collapsing. His cortisol levels at the time were described as “low”. Following the synacthen test, the results showed that the basal cortisol level was 43.81nmol/L and the cortisol level after 30 minutes was 41.27nmol/L. According to the guidelines, this patient is suffering from adrenal insufficiency. This diagnosis is determined since the basal cortisol level is below 180nmol/L plus there is no increase in cortisol levels 30 minutes post dose (there is a decrease even!), therefore remaining below normal cortisol levels.
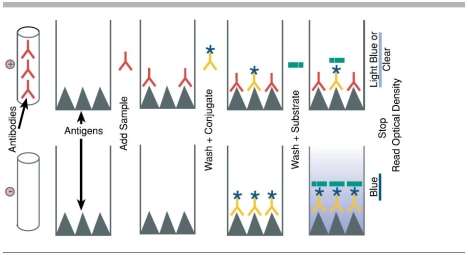
This practical used a sandwich ELISA method, a sandwich ELISA uses a primary antibody (capture antibody) coated on a plate, this antibody binds to the antigen present in the sample,in this case the antigen is cortisol. The antigen is then bound to a secondary antibody (detection antibody). In other words, the antigen is “sandwiched” between the antibodies (see figure 2 step 3). This can only be done if the antigen has at least two antigenic epitopes that can bind to the antibodies. This method has various advantages compared to other ELISA techniques such as the direct method. For example, the sandwich method does not require for the sample to be purified before analysis, also, this technique offers a high sensitivity compared to the direct ELISA technique.
Figure 2. Sandwich ELISA schematic (http://www.elisa-antibody.com/ELISA-Introduction/ELISA-types/sandwich-elisa)

As shown above the antibody in step 1 is the capture antibody which is coated to the plate prior to the addition of the sample. Step 2 shows the antigen present in the sample binding to the specific capture antibody before being bound to the detection antibody in step 3. Step 4 and 5 show the addition of the secondary antibody which binds to the detection antibody to then create a coloured signal when in presence of the correct substrate. This signal is then measured spectrophotometrically.
Another widely used ELISA method is the competitive method. This method is different to the sandwich ELISA since the detection of the antigen or antibody is done in a different approach. The main advantage of using a competitive ELISA is that an antigen can be detected even though the sample is crude or impure.
Figure 3. competitive ELISA schematic (http://www.elisa-antibody.com/index.php?page=competitive-elisa)
It works on the principle that the more antigen/antibody present in the sample, the less coloured signal will be produced. The antigen or antibody bound to the plate will bind to the specific antibody or antigen present in the sample. A detection antibody is then added, this detection antibody will only bind to the antigen or antibody that was originally bound to the plate, and cannot bind to the antigen/antibody from the sample (it is specific to the plate-bound antigen/antibody). Therefore, the added antibody/antigen and the antigen/antibody present in the sample are in competition for the plate-coated antigen or antibody. Only the detection antibody added after the sample will produce a coloured signal in presence of the correct substrate. This creates a change in intensity of the coloured result depending on the amount of antigen/antibody in the sample. The amount of antigen/antibody in the sample will decrease the intensity of the signal.
This practical used wells coated with monoclonal antibodies. Monoclonal antibodies are identical antibodies produced from a single type of B cell. These identical B cells produce antibodies that present only one unique epitope. These cells are isolated and grown to multiply the production of these single epitope antibodies. The characteristics of these monoclonal antibodies offer a very high specificity since there is only a single epitope, minimizing cross reactivity with different epitope presenting antibodies. These antibodies are preferred in assays which require quantification due to the high specificity.
Another type of antibody that can be used in immunoassays are polyclonal antibodies. These antibodies, unlike monoclonal antibodies, originate from various types of B cells. They can recognize more than one epitope of an antigen or antibody. Technically, individual polyclonal antibodies are monoclonal antibodies, they therefore have the same characteristics as mentioned earlier. They can bind to specific epitopes on an antigen/antibody. But since there are many different types of monoclonal antibodies present (polyclonal), the antigen can be detected by binding the antibodies to different epitopes present on the antigen. Polyclonal antibodies have various advantages, for example, production is less expensive and quicker than producing monoclonal antibodies since the specificity is not as important. The use of polyclonal antibodies is preferred in assays that require less specificity, more robustness, stability, sensitivity for detecting small amounts of antigen and time constrained protocols.
Conclusion
Immunosorbent assays are widely-used in clinical laboratories to detect compounds in samples. They are highly sensitive, specific and reproducible which makes them a great tool in a clinical laboratory. There are different types of enzyme linked immunosorbent assays (ELISA), each with its own way of detecting the antigen/antibody. The use of monoclonal and polyclonal antibodies varies depending on the antigen/antibody needing to be detected and they both come with their own advantages and disadvantages. One of these ELISA methods can be used to detect and measure cortisol levels in serum samples. By performing a synacthen test, a set of serum samples from a patient can be drawn before and after administering a dose of synthetic ACTH. By measuring cortisol levels in these samples, an assessment of adrenal functions can be made. This test helps diagnose adrenal insufficiencies and disorders related to it.
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more