Five-Antiretroviral Drug Class Resistant HIV-1 In A Treatment Naïve Patient Suppressed With Optimized Antiretroviral Selection
Joseph M Volpe, Douglas J Ward, Laura Napolitano, Pham Phung, Jonathan Toma, Owen Solberg, Christos J Petropoulos, Charles M Walworth
Abstract
Transmission of HIV-1 exhibiting reduced susceptibility to protease and reverse transcriptase inhibitors is well-documented, but is evolving for integrase inhibitors and is limited for the fusion inhibitor enfuvirtide. We describe here a case of transmitted 5-drug class resistance involving protease, reverse transcriptase (nucleoside and non-nucleoside), integrase, and fusion inhibitors in an antiretroviral naïve patient that subsequently was successfully treated based on the optimized selection of an active antiretroviral drug regimen.
Keywords:
Transmitted Drug Resistance; TDR; Integrase Inhibitor Resistance; Tropism;
Introduction
Drug susceptibility can be a key determinant in choosing an initial antiretroviral (ARV)regimen for patients who are naïve to ARV therapy.The selection of aregimen in which individual components have less than full susceptibility can result in virologic failure.Transmission of HIV-1 exhibiting resistance to protease (PR) and reverse transcriptase (RT) inhibitors is well-documented[1-3] andbecause of this,DHHS guidelines recommend baseline genotypic resistance testing to guide drug selection in patients who are ARVnaïve[4]. However, given the relative newness of the integrase (IN)inhibitor class and the limited use of enfuvirtide (ENF), transmitted resistance for these drug classes is less well-defined[5-7]. To date, two cases of raltegravir resistant HIV-1 transmission have been reported in the literature[5-6].
Although transmission of virus resistant to more than one ARV class occurs less frequently than a single class[2-3], when it occurs, the selection of a baseline regimen can bemore challenging. Such was the case in 2005 when a New York City man acquired a dual tropic, multidrug-resistant HIV-1 strain[8], during a time when fewer therapeutic options were available. Here, we describe the first documented multidrug-resistant HIV-1 strain containing variants that exhibit resistance to five ARV classes. This reportnot only demonstrates one of the earliest cases of transmitted resistance to the integrase strand-transfer inhibitor (INSTI) class, but also exemplifies the need to develop a detailed resistance profile prior to initiating therapy.
Case History
A man in his forties was hospitalized in 2010 with severe flu-like illness. HIV-1 antibody testing during hospitalization was negative. HIV-1 RNA testing by PCR was not performed. Six months later, follow-up HIV-1 antibody testing was positiveand infection was confirmed by Western blot. Initial CD4+ count and viral load were 376 cells/mm3 and 211,540 copies/mL, respectively. Baseline genotypic resistance testing demonstrated extensive resistance to nucleoside and non-nucleoside reverse transcriptase inhibitors (NRTI, NNRTI) as well as protease inhibitors (PI) [Figure 1]. Confirmatory testing was performedto verify the initial genotypic resistance profile. Additional genotypic testing for INSTI resistanceand phenotypic testing for PI, NRTI, NNRTI and INSTIsusceptibility was conducted. Phenotypic co-receptor tropism testing was also performed to evaluate additional ARV treatment options. Due to the complexity of the baseline resistance profile, ENF susceptibility was also assessed.
Methods
Resistance-associated mutations (RAMs) to inhibitors of HIV-1 PR, RT, and IN were identified by conventional DNA sequencing(GenoSure® MG, GenoSure®Integrase, Monogram Biosciences and LabCorp).Phenotypic susceptibility to PR, RT,and INinhibitors, ENF, and co-receptor tropism were also assessed using well-established pseudo-virus infectivity assays (PhenoSense®, PhenoSense GT®, PhenoSense®Integrase , PhenoSense® Entry, and Trofile®, respectively; Monogram Biosciences). Molecular clones of full-length envelope sequences were generated and evaluated for ENF susceptibility and co-receptor tropism (PhenoSense® Entry, Trofile®). The gp41 sequences of envelope clones were generated by conventional DNA sequencing. Phylogenetic analysis was conducted on clonal gp41 sequences to rule out co-infection.
PR and RT regions were also interrogated by massively parallel (“deep”) sequencing. A sequence library was generated using the IlluminaNextera XT library preparationkitand an IlluminaMiSeq2x250bp paired-end run resulted in 1,017,032 reads with an average read depth of >15,000X (after alignment). Reads were trimmed using cutadapt and fastx toolkits and aligned to the NL4-3 reference genome (accession number AX032749.1) using bowtie2[9].
Results
Genotypic resistance analysis of the baseline virus identified mutations associated with resistance to PI (L10Y, K20I, E35D, M36I, K43T, I62I/V, V82K), NRTI (M41L, D67N, L74V, L118I), and NNRTI (K101E, Y181C, V189I, G190S) [Figure 1]. Repeat genotypic resistance analysis from a second draw confirmed the initial findings, as well as mutations associated with INSTIresistance (G140S, Q148H). Reductions in susceptibility to PI, NRTI, NNRTI, and INSTI were confirmed by phenotypic testing, which demonstrated large reductions in susceptibility to efavirenz, nevirapine, and raltegravir. Notably, the NRTI resistance mutation M184V was not identified bygenotypicassessment although a phenotypicassessment revealedmodest reductions in susceptibility to emtricitabine and lamivudine (IC50fold change (FC) 7.16 and 5.25 respectively)that exceeded the biological cutoff (FC 3.5).Further analysis of the sample using deep sequencing failed touncover minor variants harboring an M184V substitution. Neither deep sequencingnor the phylogenetic analysis of envelope clone sequences yielded evidence for dual infection.
Initial phenotypic analysis for ENFsusceptibility (FC 6.31)fell within 0.2 log10of the biological cutoff(FC 6.48). This cutoff is based on a reference population of ENF-naïve baseline isolates from the TORO clinical trials [10]. Given the proximity of the measuredENF susceptibility of the patient sample to the biological cutoff and considering both the broad distribution of the susceptibility of the reference population and the inherent variability of the phenotypic assay (±3-fold), further analysis was warranted. Consequently, envelope gp41 sequencing was performed on 44 molecular clones generated from the virus population. Phenotypic analysis to determine ENF susceptibility was performed on 20 of the 44 clones that had unique gp41 sequences relative to the consensus sequence of the virus population. Two clones, (#10 and #25), exhibitedreducedENF susceptibility(FC 46 and >MAX, respectively), well above the biological cutoff. The gp41 sequence of these two clonesrevealed novel substitutions (Q40R, N43S) at amino acid positions previously associated with ENFresistance [11]. Co-receptor tropism testing indicated that 19 of the 20 selected clones exhibited R5 tropism, consistent with the R5 tropism determination for the overall virus population.
Based on resistance and co-receptor tropism testing, the patient was placed on a regimen of ritonavir-boosted darunavir, tenofovir/emtricitabine, and maraviroc, which successfully suppressed viral replication to <200 copies/mL at 2 months that was maintained one year post-initiation of treatment. CD4 count improved from 376 cells/mm3 at diagnosis to 614 cells/mm3 at one year of treatment. At three years post-initiation of treatment, the patient’s virus remains suppressed with a CD4 count of 865 cells/mm3 (Table 1).
Discussion
To our knowledge, this case representsthe first confirmed report of the transmission of HIV-1 containing variants exhibiting resistance to five antiretroviral drug classes,as well as the third confirmed report of transmitted INSTI resistant HIV-1.The selection of tenofovir/emtricitabinein the treatment regimen was based upon an assumption that anM184V variant might have been present below the limit of detection for population sequencing. Often, lamivudine or emtricitabineis incorporated intoARV treatment regimens toexploit the impaired replication of M184Vvariants, despitelimited evidence to support this approach. Detectable reductions in phenotypic susceptibility due to M184V variantsrequire a viral subpopulation approximating 40% of the total viral population[12]. Here, the absence of an M184V-containing subpopulation below the limit of detection of genotypic assays was confirmed by deep sequencing.Thus, the observed reduction in phenotypic susceptibility to emtricitabine and lamivudine waslikely due to the combination of L74V and V118I substitutions along with the thymidine analog substitutions M41L and D67N.
This case further demonstrates the clinical utility of co-receptor tropism testing to guide maraviroc prescription.ARV treatment experienced patients have a lower prevalence of R5 tropic virus compared with ARV treatment naïve patients. Laboratory studies demonstrate preferential transmission of R5 virus[13] and data from clinical cohorts demonstrate that over 70% of ARV naïve patients harbor R5 tropic virus[14]. In ARV treatment naïve patients, there is no genetic linkage data to suggest that ARV resistant profiles in pol influence envelope co-receptor tropism. Despite the extensive ARV resistance profile identified within the pol gene, the case patient was treatment naïve and thus more likely to harbor R5 tropic virus.
Envelope substitutions associated with ENFresistance include Q40H and the N43D.Clonal analysis of this case virus led to the identification of two novel substitutions Q40Rand N43S that were demonstrated to confer high level phenotypic resistance to ENF.
The value of baseline resistance testing to determine an optimal ARV treatment regimen is highlighted in this case report. Current DHHS guidelines recommend supplemental genotypic INSTI resistance testing when transmitted INSTI resistance may be a concern[4]. There is evidence that transmitted INSTI resistance is followingthe same temporal coursepreviously observed for NRTI, NNRTI and PI[15]. With the recent approval of a third INSTI, more widespread INSTI use, overlapping INSTI cross resistance profilesand documentation of this third case of transmitted INSTI resistance, baseline testing for INSTI resistance may become prudent.
Figure 1:
Results of both the genotypic and phenotypic drug resistance analyses are listed here. The net assessment column considers both the genotype and phenotype test results and provides a final resistance call based on the cumulative data.
† Single values represent biological cutoffs, and ranges indicate lower and upper clinical cutoffs.
‡ Fold change is defined as the ratio of the measured IC50 for the patient-derived virus to that of the NL4-3reference virus.
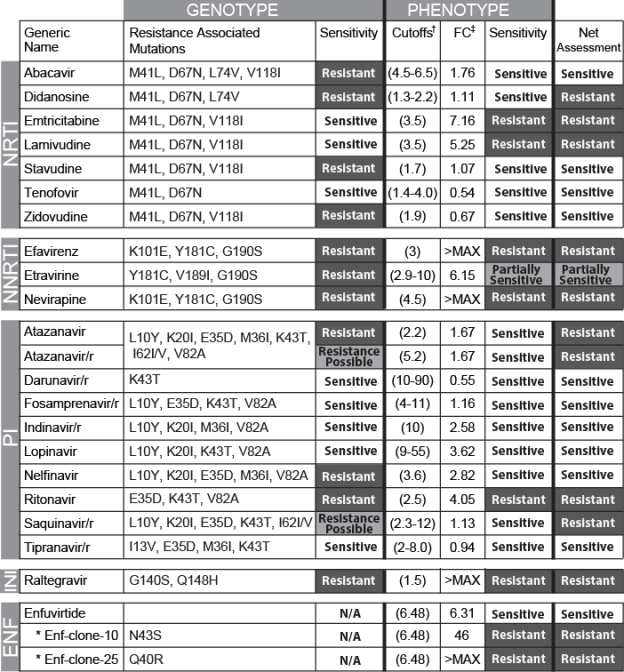
Table 1: Patient Clinical Parameters
|
Month/Year |
Viral Load (copies/mL) |
CD4 (cells/mm3) |
CD8 (cells/mm3) |
CD4/CD8 Ratio |
|
7/11 |
211,540 |
376 |
1571 |
0.24 |
|
12/11 |
60 |
381 |
966 |
0.38 |
|
3/12 |
80 |
385 |
785 |
0.49 |
|
7/12 |
40 |
614 |
1000 |
0.61 |
|
11/12 |
< 75 |
704 |
934 |
0.75 |
|
2/13 |
< 75 |
619 |
808 |
0.77 |
|
6/13 |
< 75 |
774 |
834 |
0.93 |
|
12/13 |
< 75 |
725 |
707 |
1.03 |
|
5/14 |
< 75 |
865 |
888 |
0.97 |
Viral loads, CD4 and CD8 counts, and CD4/CD8 ratiosfor this patient are listed over the treatment period. The initial viral load measurements were obtained using the Roche COBAS® TaqMan® 2.0until 7/12. Subsequent values were obtained using the Siemens Versant® HIV-1 Branched DNA assay.
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more