Abstract
Studies in recent years have shown that combustion-derived particulates have a significant effect on global climate change. This project explores the use of Laser Induced Incandescence (LII) as a qualitative measurement technique for combustion-derived soot. The methodology behind LII is reviewed, discussing its position compared to alternative soot measurement techniques. Details are included on the effects of laser profiles, fluence levels and the difference between running LII at the fundamental (1064 nm) or secondary (532 nm) harmonic. An experiment was set up at Cardiff University’s Gas Turbine Research Centre (GTRC) using a Litron Nano S PIV Class 4 laser to provide the excitation optics at 532nm. An Andor iStar ICCD camera was used to provide the detection optics, and a Palas GfG 1000 particle generator to simulate soot production. Although no detection filter was used, the experiment successfully demonstrated the principles of a functioning LII system. It was subsequently concluded that the internal optics of the laser should be modified to emit 1064nm light. Therefore, eradicating the need for a 532 nm detection filter and concluding that quantitative measurements can take place in future experiments.
Table of Contents
1.0 Introduction
2.0 Literature Review
2.1 Background
2.2 Environmental Effects of Soot
2.3 Health Effects of Soot
2.4 Regulations
2.5 Soot Formation Process
2.6 Types of Flames
2.7 Types of Burners
2.8 Soot Measurement Techniques
2.8.1 Laser Induced Fluorescence (LIF)
2.8.2 Extractive Laser Induced Incandescence (LII)
2.9 Laser Induced Incandescence (LII)
2.9.1 LII Background
2.9.2 LII Process
2.9.3 Laser Profiles
2.9.4 LII Theory
2.9.5 Laser Excitation Wavelengths
2.9.6 Conventional LII Set-up
2.9.7 In-situ LII Set-up
3.0 Previous Experiment Summary
3.1 Previous Set-up
3.2 Previous Procedure
3.3 Previous Results
4.0 Installation of LII System at the GTRC
4.1 The Laser
4.2 Location
4.3 Detection Optics
4.4 Soot Source
5.0 GTRC Experiment
5.1 Procedure
5.2 Results and Discussion
6.0 Conclusions and Future Work
7.0 References……………………………………………………..
Appendix A
As the demand for energy in the world today increasing exponentially, there is an urgency to reduce the particulate emissions generated by methods at which we produce this energy. To improve our long-term health and prevent further climate change there is a significant push towards renewable energy sources. Even so, energy production from fossil fuels continues to dominate the energy sector. Consisting of unburnt hydrocarbons, NOX, SOX, CO and particulate matter, it is essential that we better understand and control these unwanted emissions.
Particulate matter is coming under closer scrutiny as a pollutant. Particles as small as 2.5 m are known to produce adverse health effects and are suspected of producing high altitude clouds which adversely affect the earths climatology (Snelling et al. 2000). These particles can deposit themselves on the human lungs and any particles smaller than this can find their way in into the lining of the blood within the lungs which allow the oxygen and carbon dioxide to move between the lungs and the bloodstream. Soot is one of the most significant of these emissions and is hence becoming more tightly controlled by international regulators e.g. ICAO. In order to develop methods to limit soot production we must first develop suitable means for measuring its formation, emissions and other related parameters.
There are a range of methods currently used for the measurement of particulate matter. The method of soot measurement and quantification that this study will be focusing on is Laser Induced Incandescence (LII). What was an emerging technology is now used in a wide range of experiments to temporally and spatially measure both primary particle size and soot volume fraction. A conclusive discussion of the LII process will be detailed later.
The aim of this project is to develop a functional LII system for the qualitative analysis of soot at the Cardiff University Gas Turbine Research Centre (GTRC) in Port Talbot. Although the primary focus of study is intended for the GTRC, the system should have an element of portability allowing it to be used in a range of applications. As shown in the literature (Section 2.9) and due to the numerous hardware options available within Cardiff School of Engineering, there are multiple viable approaches that may be pursued towards the development of a functioning LII system. As such to guide the design direction, first a comprehensive literature review was undertaken.
Soot is essentially a condensed-phase particulate formed in the combustion of hydrocarbons (Wang 2011). Due to its radiative heat transfer properties, soot formation can be desirable in some combustion scenarios, heat furnaces and boilers rely on the formation of soot to operate efficiently. However, it is usually thought of as an undesirable combustion product which impedes the efficient use of hydrocarbon fuels (Santoro et al. 1983).
When incomplete combustion occurs in an internal combustion engine the soot formed collects on the inside walls of the engine and the thermal absorption of soot can cause a significant reduction in the working efficiency of the engine (van Setten et al. 2001).
These well documented negative effects of soot only further confirm the importance of accurately measuring and regulating the soot currently produced in the world today. Commercial methods for measuring the concentration of soot have been around for decades resulting in limited scope and accuracy, often producing time-averaged results which are difficult to apply to common scenarios without modifications. Non-intrusive optical measurement techniques have potential in solving these problems (Kempthorne 2010).
In recent years, soot has developed an increasing amount of recognition by scientists as having the potential to contribute significantly towards climate change. As soot particles absorb sunlight they heat the surrounding air, reducing the amount of sunlight reaching the ground and resulting in a cooler surface temperature (The Environmental Literacy Council 2015). An unstable atmosphere is a result of the rising heated air, forming clouds and ultimately rainfall to areas with a heavy concentration of soot formation. The primary component of soot is black carbon, defined as (US EPA 2012 p. 17):
“A solid form of mostly pure carbon that absorbs solar radiation (light) at all wavelengths. Black carbon (BC) is the most effective form of particulate matter (PM), by mass, at absorbing solar energy. BC is a major component of “soot”, a complex light absorbing mixture that also contains organic carbon (OC)”.
Black carbon has numerous characteristics which differentiate it from long-lived greenhouse gases (GHGs) like CO2, such as a short atmospheric lifespan and strong absorption capabilities. Other unwanted pollutants are produced from the same combustion processes that generate BC emissions (US EPA 2012), most notably OC which is formed from gaseous precursors such as SO2, NOx and volatile organic compounds (VOCs). An additional key component of soot, organic carbon is defined as (US EPA 2012 p. 21):
“OC refers to the mixture of compounds containing carbon bound with other elements like hydrogen or oxygen. OC may be a product of incomplete combustion, or formed through the oxidation of VOCs in the atmosphere. Both primary and secondary OC possess radiative properties that fall along a continuum from light-absorbing to light scattering”.
BC is a form of primary particulate matter as it is emitted directly from the combustion source, this differentiates it from secondary particulate matter including a range of OCs. A diagram displaying the effects of BCs on climate change compared to GHGs is featured in Figure 2.1 (US EPA 2012).

Figure 2.1 Effects of Black Carbon on climate change in comparison to Green House Gases (US EPA 2012).
Soot mixtures vary in composition using a combination of ratios for OCs to BCs, in addition to other inorganic compounds. Compounds of soot combine with moisture to form acid rain. The quality of water in both lakes and rivers are dramatically reduced due to acidification, in addition to damaging soil and crops, and changes nutrient balances in various ecosystems (Keefe 2013). Additionally, these volatile compounds that form acid rain are directly associated with the haze that is formed when sunlight interacts with small particles in the atmosphere (Weidman and Marshall 2012). Smog, another local air quality issue is also associated with soot production.
Soot poses a significant threat to public health, due to the size of particulate matter it can easily enter your lungs, bloodstream and ultimately cause damage to the human body in a range of different ways. One metric of soot is PM 2.5, denoting that the particulate matter is 2.5 micrometres (m) in diameter or smaller. Such fine particles are even smaller than dust particles, or approximately 1/30 of the size of a human hair. Combustion generated soot from an internal combustion or gas turbine engine is an order of magnitude smaller than this and is typically ultrafine (<100 nm). Figure 2.2 displays the relative size of a soot particle (Johnson 2015).

Figure 2.2 Diagram showing the size of a combustion soot particle (<0.1m) compared to other known quantities (Johnson 2015).
There is considerable variation in morbidity and mortality rates associated with exposure to PM 2.5. Short term exposure to levels that commensurate with current US regulations for maximum 24 hour levels results in an increase of approximately 1 – 3% in non-accidental deaths. Furthermore, Kennedy (2007) highlights longer term exposure to a point which meets annual concentration limits can cause an increase in non-accidental deaths of up to 5%, with this figure increasing further if co-pollutants such a CO and NOx are present.
Soot particles in the air are a major contributing factor towards respiratory diseases. The fine particles are the most hazardous in terms of lung damage due to their ability to penetrate deep into the air passages. Larger particles (greater than 3m) are trapped in the nose and throat from which they are easily eliminated, but finer particles can stay intact for years in the inner most regions of the lungs, which have no effective mechanism for particle removal. “The lodged particles in the lungs can cause severe breathing trouble by physical blockage and irritation of the lung capillaries” (Srinivasarao 2013 p. 82).
The presence of toxic compounds in the deepest part of the lungs can lead to severe bronchial problems and increase one’s susceptibility to infections such as asthma, bronchitis and pneumonia. In addition to these problems, soot particles present in the air can cause underlying health problems such as congestive heart failure to deteriorate. In the United States, data has also shown that soot annually causes almost 300,000 asthma attacks and 2 million lost workdays, also due to respiratory problems (Keefe 2013).
Health studies have linked a range of cancers, most notably lung cancer, to both the carbon soot particles produced by diesel engines, and more specifically emitted aldehydes and polycyclic aromatic hydrocarbons (PAHs). Inhalation is not the only transport medium in which toxic compounds can enter the human body. Exposure can also occur via ingestion and absorption through the skin, the latter resulting in an increased chance of skin cancers (National Cancer Institute 2015).
Combustion-generated soot particles clearly pose a significant risk to both human health and the environment, as a result in recent years there has been a mounting pressure from governments imposing far more stringent regulations as to its production. Significant changes have been required to both the quantity and compositions of harmful soot particles as a direct result of the tighter regulations. It is for this reason that there has never been a more important time in history than now to accurately detect and quantify the levels of soot being produced.
In the automotive industry, there have been continual strides to improve air quality and health since the first European exhaust emissions standard for passenger cars was introduced in 1970. Most notably was the Euro 1 standard in 1992, insisting catalytic converters were fitted to petrol cars and limiting particulate matter emissions in diesel engines to 0.14 g/km. Yet, it was not until the introduction of Euro 5 in January 2011 that particulates were limited for petrol engines. Euro 5 also introduced a limit on particle numbers for diesel engines in addition to the weight limit (Automobile Association 2015).
Euro 6 is the latest revision which was implemented in September 2015, it imposes further restrictions on petrol engines bringing limits in line with diesel. Euro 6 applies to both heavy and light duty engines, diesels achieve conformity with the use of a Diesel Particulate Filter (DPF). Currently particulate matter emissions for both diesel and petrol engines cannot exceed 0.005 g/km and 6.0×1011 particulates/km. In the period between 1992 and 2015 some pollutants have been reduced by as much as 96% (Automobile Association 2015).
In response to the adverse physical effects, the European Union (EU) has developed an extensive body of legislation which establishes the health based standards and objectives for several pollutants in the air (European Commission 2016). The legislation is built on certain principles. Firstly, the Member States of the EU divide their territory into zones to which they then undertake assessments of the air pollutions levels using the appropriate techniques. An air quality plan is then carried out if levels are too high, this ensures compliance with the limit value before the date when this value comes into force. Finally, the information gathered on the quality of air should be made accessible to the public. The latest update to the EU air quality standards concerning PM 2.5 was issued in 2008, introducing a new PM 2.5 objective for public exposure of fine particles. This latest directive is displayed in Table 2.1.
“These objectives are set at the national level and are based on the average exposure indicator (AEI). The AEI is determined as a 3 – year running annual mean PM 2.5 concentration averaged over the selected monitoring stations in agglomerations and larger urban areas, set in urban background locations to best assess the PM 2.5 exposure to the general population” (European Commission 2016).
Table 2.1 2008 EU directive for PM 2.5 (European Commission 2016).

In a pre-mixed fuel-air system, under ideal conditions, the combustion of hydrocarbons is termed stoichiometric where by the reactants are burnt entirely leading to the production of carbon dioxide and water. In combustion devices such as Industrial Furnaces, Internal Combustion Engines or Gas Turbines, conditions deviate from being ideal. Incomplete combustion occurs resulting in the production of unwanted products such as Carbon Monoxide, Hydrogen and Soot.
Soot Formation occurs when the ratio of carbon to oxygen, (C/O)cr, drops below 1 (Mansurov 2005), often referred to as fuel rich combustion where there is an insufficient amount of oxygen for the fuel to react entirely. The ratio of carbon to oxygen depends on the fuel type, thresholds of soot formation points for a range of fuel compositions are shown in Table 2.2 (Haynes and Wagner 1981).
Table 2.2 Soot formation thresholds of carbon to oxygen for premixed flames (Haynes and Wagner 1981).

Pressure and temperature are key factors when considering soot formation thresholds within a flame, soot is formed when the carbon to oxygen ratio is achieved for a given pressure (Mansurov 2005). When there is a pressure rise, the minimum (C/O)cr ratio is moved towards being a stoichiometric mixture (C/O = 0.33 for a C2H4 – air mixture) (Mansurov 2005). At low temperatures, the carbon to oxygen ratio increases as the temperature decreases. “Below 1350 – 1400 K, the burned gas contains a substance with a high molecular weight, but at such a low temperature, it cannot be transformed to soot for a time of contact t < 0.1 sec.” (Mansurov 2005 p. 728).
Following the initial reaction between the oxygen and fuel molecules many different structures are formed, it is generally considered that a polycyclic aromatic hydrocarbon (PAH) is the primary precursor to soot formation. A breakdown of the formation of PAHs is displayed in Figure 2.3 (Bockhorn 1994). Chain structures consisting of carbon atoms along with a range of hydrocarbon structures are formed in the initial stages of combustion. For fuels without an original aromatic ring structure, the first cyclic molecule is formed via hydrogen detachment (Marinov et al. 1999).
PAH structures require a period of development before becoming fully fledged soot particles within the flame as shown in Figure 2.3. Further particle growth is reliant on two mechanisms; surface growth and coagulation. Surface growth is when hydrogen detachment occurs on the surface due to reactions between the PAH radicals and the primary cluster. Instead of increasing the size of the cluster, generally these reactions increase the primary particle size (Richter and Howard 2000). Coagulation is where larger collisions occur between more evolved soot particles, if the soot is still young then a roughly spherical shape is maintained (Richter et al. 2004).

Figure 2.3 Schematic diagram of soot formation in a pre-mixed laminar diffusion flame (Bockhorn 1994).
There exists two general classifications for flame types which distinguish how the fuel and oxidiser interact when combustion occurs within the flame front (thin cross section of the flame). In a premixed flame, the fuel and oxidiser are mixed before an ignition source is introduced to initiate the reactions. Diffusion flames, on the other hand, have separate fuel and oxidiser regions and rely on natural convection movements caused by concentration and temperature gradients to allow combustion to occur (Kempthorne 2010).
Due to the nature of a premixed diffusion flame, whereby the oxidising agent is evenly spread throughout the volume, the speed of combustion is entirely dependent on the time taken for the individual combustion processes. On the other hand, diffusion flames are limited by the speed of the gas diffusion process. Heat release and reaction rates are determined by the mixing rate.
As diffusion timescales are typically much longer than the scales of most combustion reactions, diffusion flames have a wider flame front and burn slower compared to a pre-mixed flame under similar conditions (Turns 2006). Diffusion flames have been used extensively for scientific research because they contain the processes that affect soot formation in real fuels. Thus, diffusion flames are ideal for an extensive study of soot formation (Kholghy 2012). A steady laminar co-flow diffusion flame is the simplest two-dimensional diffusion flame and is used in a wide range of combustion studies. The soot formation process in this type of flame is displayed in Figure 2.4 (Turns 2006), a non-smoking flame is shown on the left while a smoking flame is illustrated on the right. The wings of the flame are where the soot inception zone occurs, this is where there is the highest concentration of fuel.

Figure 2.4 Typical locations of soot formation in a laminar diffusion flame (Turns 2006).
Within the combustion community there are three ‘target flames’ which are commonly used to investigate flame properties; a McKenna, Gülder and Santoro burners. The conditions for an ideal sooting flame for each of these is displayed in Table 2.3 (Schulz et al. 2006).
Of the three types, the McKenna flame produces the lowest soot volume fraction. It functions via a partial plug located directly above the fuel outlet to produce a thin, axially symmetric flame. It is common to fix a metal plate above the nozzle exit to enhance flame stability, 21mm above in the case of Axelsson et al. (2000). A gradual pre-heating effect can occur if the burner is left on for longer experiments, active cooling is often required to remedy this by keeping the base of the nozzle at a constant temperature.
The Santoro flame, although a strong soot source, requires a chimney above to provide stability. As a result, the reproducibility of this setup in a variety of labs is questionable (Schulz et al. 2006).
Table 2.3 3 Types of target flames and measurement conditions (Schulz et al. 2006).

The Gülder burner is a popular type which produces a strongly sooting flame and is best suited as a stable target flame (Schulz et al. 2006). This burner consists of a central fuel nozzle surrounded by a co-flow stream of gas, this surrounding flow of air must pass through a series of ceramic beads before reaching the central nozzle. The purpose of the beads is to remove any unwanted vortices that may be present in the air flow with the aim of making it easier to establish a laminar flame. A cross section of the burner is presented in Figure 2.5 (Kempthorne 2010), an image of the resulting flame is then displayed in Figure 2.6 (Smallwood and Schulz 2005).

Figure 2.5 Cross section of a Gülder burner (Kempthorne 2010)

Figure 2.6 Resulting target flame of a Gülder burner (Smallwood and Schulz 2005)
Nepf (2002) states that fluorescent molecules absorb light at one frequency and then re-emit (fluoresce) light at a different frequency. Then goes onto discuss that during experiments the molecules are excited by a laser light whose frequency closely matches that of the molecule. As the frequency of the fluoresced light is different to the excitation light, the latter can be filtered out so specific planes within the flow can be displayed.
The LIF technique is based on the production of C2 radicals from laser vaporised soot. The laser wavelength is chosen so that besides vaporising the soot, it excites the C2 radicals, and the subsequent fluorescence signal is detected (Bengtsson & Aldén 1995). Vaporisation of soot particles occur at laser intensities of around 107 W/cm2, an intensity of 1 – 2 magnitudes above this value can easily be obtained with a conventional Nd:YAG and dye-laser system.
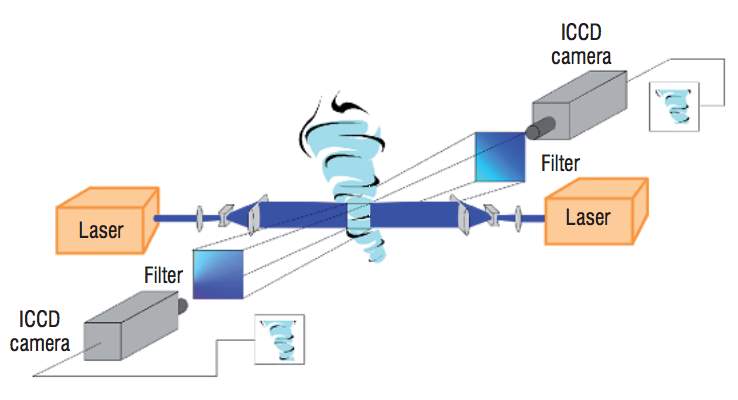
Figure 2.7 Diagram of a typical experimental set-up for PLIF (Grisch & Orain 2009).
When a system of cylindrical and spherical lenses are used to focus the laser beam into a thin sheet, the technique is commonly referred to as Planar Laser Induced Fluorescence (PLIF). Fluorescence from part of the laser sheet interacting with the flow is collected by an imaging lens at a right angle, and imaged onto a gated and intensified CCD camera. A spectral band-pass filter is placed in front of the imaging lens to reject unwanted light and select the suitable fluorescence. The spatial resolution achievable is dependent on the pixel size of the detector array, the depth-of-field of the collection optics, the image magnification factor, and the size of the beam waist at the probe volume (Grisch & Orain 2009). An example of a PLIF set-up is shown in Figure 2.7, in this case there are two gated Intensified Charge-Coupled Device cameras (ICCD). In that particular set-up there are two lasers exciting two different transitions in order to counteract an inherent sensitivity to temperature. They are being fired sequentially as to temporally separate the fluorescence decays of each transition.
There is currently a PLIF system being run by Cardiff University at the Gas Turbine Research Centre (GTRC), which has a gas turbine combustor simulation providing full optical access. It is thought that this system can be optimised to provide simultaneous LII and PLIF. Currently there is an Nd:YAG laser coupled to a tunable dye-laser which is directing unwanted 1064nm light towards a beam dump which instead could be utilised for LII.
In an extractive LII system, a sample of soot is extracted from the exhaust of the source under investigation. The sample is then transported to an LII measurement system typically consisting of an Nd:YAG laser and photodetector set up. In a traditional in-situ LII set up, the conditions where the experiment is taking place can be challenging because of extremely high temperatures. Therefore, the implementation of access for both the laser and optical detection devices can prove difficult. It is for this reason that extractive LII can prove advantageous as it does not require direct access to the region being measured.
As a direct result of transporting the sample, particle losses occur and subsequently the soot volume fraction of the sample in the measurement device is not aligned to the combustion source. Soot has an inherent tendency to deposit itself on any surface it encounters, just as it would on the inside walls of an internal combustion engine.
The study of Laser Induced Incandescence (LII) has taken place for over a quarter of a century and a great number of advances have been made in this time. Already a proven technique for qualitative measurement, it is a popular quantitative measurement tool for primary particle size and soot volume fraction. A high energy, short pulsed laser is used to rapidly heat particulate matter to the point where they emit visible light. The temperature decay of the individual soot particles is then studied to determine the concentration and particle sizes. During the process the soot particles are modelled as black bodies which by nature are strong absorbers of radiation.
In theory LII can be demonstrated with any combustion system generating an aerosol which contains non-volatile absorbing particles (Black et al. 2008). The process was first introduced by Weeks and Duley (1974). Black et al. (2008) further explains that the potential of LII for study of sooting flames was quickly realised by the combustion diagnostics community, ultimately leading to the production of the first LII process model (Melton 1984). This model has since been refined considerably.
Traditionally the study of LII has been applied to sooting laminar diffusion flames as discussed in Section 2.7. The first diesel engine in-exhaust application was published by Case and Hofeldt (1996), with the first studies on aero gas turbine exhausts following soon after. For an ideal non-intrusive measurement, airflow should not be disrupted by the LII apparatus and as a result should not impede performance. When a traditional LII configuration is used for flames a light sheet is formed from the laser beam and the incandescent light is collected perpendicular to it (Black et al. 2008). In larger engine exhausts, experiments often adopt a ‘backward configuration’ LII set-up where light is collected along the laser beam path (Black et al. 2008).
The general principle of LII is relatively straightforward as it involves looking at the temperature decay of the individual soot particles to determine the concentration and primary particle size. However, the actual method can be very complex meaning several experimental issues should be considered (Kempthorne 2010).
The laser itself is pulsed for up to 20 nanoseconds to heat up the particulate matter from room temperature to almost soot sublimation temperature (~4000K). The soot particles incandesce at this point, the characteristics of these radiative emissions are then picked up by one or more photo detectors with a signal then recorded. The magnitude of the LII signal is dependent on the volume fraction of particles in the detection region, and the decay rate of the LII signal is mainly governed by the specific surfaces of the particles, which in turn depends on the primary particle size (Schulz at al. 2006).
After the initial heating, the LII signal will gradually decay over time as the particles begin to cool down, images are then taken at two pre determined moments in order to find the primary particle size of the soot. By their very nature the larger particles will be slow to cool down where as smaller ones will be much faster. As can be observed in Figure 2.8 (Smallwood 2009), once sublimation ends the predominant form of heat loss is through conduction to the surrounding gas. Radiation is essential in order to produce the LII signal, although the quantity of heat loss through this medium is significantly less compared to conduction and is therefore irrelevant in terms of heat transfer (Smallwood 2009). Due to the presence of unwanted evaporation in the first 100ns, the first image must be taken after this period in order to obtain accurate particle size measurements.

Figure 2.8 Graph illustrating effects of several heat transfer terms over time against the rate of particle temperature change (Smallwood 2009).
Nd:YAG lasers are typically used for LII set ups due to their high reliability, availability and temporal accuracy. They produce a high powered pulse with a duration of 8 – 15ns and operate at either the fundamental (1064nm) or secondary (532nm) harmonic. Lasers operating at the fundamental harmonic are often preferred as they are less likely to pick up non-LII signals. At 532nm LIF signals peak in the first ~50ns, by using a 1064nm laser this interference can be avoided.
Laser fluence has a big impact on the intensity of the received LII signal; usually quoted in J/cm2. At low fluences the peak LII signal increases gradually as particle temperature increases, therefore increasing the amount by which they incandesce. As fluence then continues to increase the LII signal begins to plateau and drop off slightly, it is at this point that we can consider the signal as being nearly independent of fluence and ideal for measurement. The fluence level where these sublimation effects are seen are ~0.2 J/cm2 for a laser operating at 532nm, and ~0.3 J/cm2 for a laser operating 1064nm.

Figure 2.9 3D Views of the three possible spatial distributions of laser energy for experimental LII setups; Top-hat, 1D Gaussian Sheet and 2D Gaussian Beam (Bladh and Bengtsson 2004).
The spatial profile of the incident laser beam must be known in order to achieve an accurate LII signal, the shape determines the extent to which the resulting signal is dependent on fluence. Figure 2.9 is a 3-D visual representation of the three possible beam profiles for an experimental LII set up; Top-hat, 1D Gaussian Sheet and 2D Gaussian Beam.
A 2D Gaussian Beam profile has often been used out of sheer simplicity but there is an inherent problem of the particles being subject to variety of different energies in a non-linear heating process. The “particles at the centre of the beam experience a much higher energy fluence than those in the side wings” (Vander Wal et al. 1995), as fluence is increased further the ‘wing effects’ become more noticeable. Figure 2.10 shows the overall effect of the LII signal varying linearly with fluence.

Figure 2.10 Normalised LII signal response of the three laser profiles, data from theoretical modelling of a 532nm laser (Bladh and Bengtsson 2004).
A 1D Gaussian Sheet profile is a relatively easy profile to achieve, made by increasing the width of the beam to produce a laser sheet (Schulz et al. 2006). A drawn-out plateau section is observed once a sufficiently high fluence is reached where the LII signal remains relatively constant with an increasing laser fluence (Figure 2.10). The result is a decrease in signal intensity because of sublimation in the centre of the beam (Smallwood 2009).
The LII signal generated from a Top-Hat profile is fluence dependent as displayed in Figure 2.10, although the power can be controlled so that the whole area experiences the same fluence level. As mentioned above, once the minimum thresholds for fluence have been reached for 532nm and 1064nm beams (0.2 J/cm2 and 0.3 J/cm2 respectively), the sublimation process will become a dominating factor and no ‘wing effects’ will be witnessed.
The theory behind LII to can be used to enhance understanding of what is happening to soot particles throughout the LII process. Beginning with studying the heat transfer through the soot particles and continuing to the measurement of soot volume fraction.
Particle size is independent of the laser light heating process associated with LII, the incandescent light that is produced is nominally volumetric when the following applies (Smallwood 2009):
As soot is formed in a laminar diffusion flame, it consists of hundreds of primary particles loosely linked together. The different heat transfer processes which apply to individual primary soot particles are; absorption, conduction, radiation, evaporation and change in internal energy. The heat transfer processes of LII can be written as an energy balance for an individual primary particle (Snelling et al. 2000) (Smallwood 2009):
qi̇= q̇a+q̇s- q̇c-q̇r
Where
q̇irepresents the rate of change of internal energy,
q̇ais the laser energy absorption by the particle,
q̇sis the heat loss due to sublimation,
q̇cis the conduction heat loss to the surrounding gas and
q̇ris the heat loss due to thermal radiation. Snelling et al. (2000) also presents the formulation of these terms which are not all detailed here. However, the laser energy absorbed by a soot particle is given by (Snelling et al. 2000) (Smallwood 2009):
q̇a=CaF0q(t)
Where
F0represents laser fluence,
q(t)is the pulsed laser temporal power density and
Cais the soot particle absorption cross-section which is expressed as (Bohren and Huffman 1983):
Ca=π2dp3E(m)λ
Where
Cais inversely proportional to the laser wavelength,
λand proportional to
E(m)and the diameter of a primary soot particle is represented by
dp. The value of
E(m)which is a function of the complete index of refraction for soot,
m, is given by (Snelling et al. 1997):
Em=Imm2-1m2+2
Where:
m=n-ik
The dispersion relationship (Dalzell and Sarofim 1969) is used to obtain the refractive index. For a wavelength of 1064nm,
m=1.63+0.7iyielding a value of 0.30 for
Em. At the secondary harmonic with a wavelength of 532nm,
m=1.59+0.58iyielding a value of 0.26 for
Em. The soot volume fraction,
fvcan then be calculated by (Snelling et al. 1997):
fv=ln(τ)λ6πL E(m)
Where
Lis the flame width and
τis the flame transmission.
In theory, any laser operating in the visible or infra-red wavelength regions can be used to generate an LII signal (Schulz et al. 2006). It has been shown that wavelengths as short as 355 and 265 nm in the ultraviolet region, with a low fluence laser, can be used to heat particles to a sufficient level for LII purposes (Rohlfing 1988). However, as mentioned the fundamental harmonic and secondary harmonics of 1064 and 532 nm are generally used for LII excitation, the main difference between the two is the extent at which non-LII signals can interfere. Broadly, for several reasons 1064 nm incident light is less affected by interference than 532 nm light or shorter.
At longer wavelengths such as 1064 nm, the production of electronically-excited C2 emissions are less pronounced (Schulz et al. 2006). Observed at 532nm, these emissions occur as the laser is pulsed and then decay relatively quickly afterwards. In addition to C2, O and OH atom emissions can also be seen at an excitation wavelength of 532 nm (Vander Wal and Weiland 1994).
A key feature of running at 532 nm or shorter is the Laser Induced Fluorescence (LIF) of PAHs which fluoresce in the visible and ultraviolet regions. The fluorescence caused by PAHs are not easily separated from the LII signal with detection filters due to the fact it is seen in a large section of the visible region. The fluorescence of PAHs is short lived at high temperatures (Schulz et al. 2006) and as with the emission of C2 particles, interference occurs during the pulse of the laser and decays afterwards.
If an LII signal is detected at 532nm there is a chance that elastically-scattered light will not be entirely blocked by the filter attached to the detector causing a disturbance to the LII signal. Rayleigh scattering is dependent on wavelength with shorter wavelengths more prone to scattering. However, Infra-red scattering has much smaller cross sections than visible scattering, meaning the use of infra-red light for excitation and detection is advantageous to discriminate against scattered infra-red light (Schulz et al. 2006). Additionally, photodetectors commonly used for LII generally have no response at 1064nm which in turn limits the interference in the infra-red region.
Generally, as part of an LII set-up there are three key components; the laser, photodetector and soot source. Figure 2.11 shows the layout developed for an LII experiment by (Kempthorne 2010), this follows a similar methodology initially developed by (Snelling 1997).

Figure 2.11 Typical LII experiment layout (Kempthorne 2010).
Laser Details
The set-up in Figure 2.11 consists of an Nd:YAG laser operating at the fundamental harmonic of 1064nm. Although an invisible infrared laser beam can prove difficult to work with, as discussed it does have the benefit of not suffering from the interference that a 532nm laser would. It is for this reason that a 1064nm is considered the laser of choice. The laser is expanded with a cylindrical lens to produce a 1D Gaussian sheet profile. The ability to directly control the power of the laser is essential to obtain the optimum fluence level, parameters such as Q-switch delay can be modified on the laser itself to achieve this (Kempthorne 2010).
However, the parameters on the laser itself are not enough to give total control of the laser, various other forms of optics are required to achieve this. The key aspect to note from Figure 2.11 is the combination of a half-wave plate and polariser, they allow the power of the laser to be attenuated and controlled to achieve the desired fluence level.
Burner Set-up
The experimental set-up in Figure 2.11 used a Gülder burner, one of the three preferred burners as discussed in Section 2.7. The burner was used to calibrate the system with the aim that once calibrated, the system can be used in any environment (Kempthorne 2010). The flow conditions were equal to what is outlaid in Table 2.3 for a Gülder burner.
Signal Measurement
Often CCD cameras are used for LII but the consensus is they are unable to fully resolve the decay curve for the lifetime of the LII signal, as such Photomultiplier tubes (PMTs) are commonly favoured. As displayed in Figure 2.11, a short-wave pass filter was used to split the signal down two channels between two PMTs. The experiment run by (Kempthorne 2010) used a method called two-colour pyrometry which requires narrowband LII filters centred at two different values; 440nm and 692nm.
An alternative to a short-wave pass filter is a beam splitter which splits the signal 50/50 on both channels. Each channel then leads to a PMT with a narrowband filter centred at a certain rating, 455.5nm each in the case of (Snelling et al. 1997).
An LII system originally developed by Rolls Royce and used by Black et al. (2008) for in-exhaust LII investigations of large aero engines is illustrate in Figure 2.12. Soot particle concentrations could then be calculated and calibrated against data gained from an atmospheric kerosene burner (Black et al. 2008).

Figure 2.12 LII set-up as used by (Black et al. 2008)
This compact set-up uses a Quantel/Big Sky CFR 400 Nd:YAG laser running near infrared at 1064nm. It has an energy meter coupled to an adjustable attenuator and produces a 10ns 400mJ pulse at a repetition rate of 10 Hz. The beam itself is directed towards a dichroic mirror and is used to reflect any visible light and transmit light at the fundamental harmonic. A box houses the LII system with a window at the outlet to prevent dirt accessing the test bed. The LII signal is collected in backwards configuration (Section 2.9.1) through a prism, then directed towards an intensified CCD (ICCD) camera via the dichroic mirror (Black et al. 2008).
The images recorded by the ICCD camera were an integration of LII signals. As stated PMTs are common with LII set-ups, however an ICCD camera is advantageous for aero-engine applications because it readily identifies if another light source is interfering with the LII signal (Black et al. 2008). The intensifier attached to the camera is in principle a faster optical shutter. A 20ns gate synchronous was used with the laser to remove interference caused by lighting in the test cell, scattered 1064nm laser light was then blocked via a notch filter positioned in front of the camera lens (Black et al. 2008).
To summarise, this section has reviewed relevant literature to inform the design of a non-intrusive in-situ LII system. The next section will provide a summary of the previous experiment by Johnston (2016).
Previously, a similar experiment was undertaken by Johnston (2016) yet was unsuccessful. The experiment was not performed at the GTRC but instead a small-scale experiment was set up at Cardiff University. It was undertaken with the intention that any issues could be ironed out prior to eventual testing at the GTRC.
The previous experiment used a Litron Nano S PIV laser and a Photron APX RS high speed camera positioned perpendicular and level to the path of the laser. Initially an excitation sheet optic was fitted, while an LII narrow-band filter was fitted to the camera lens.

Figure 3.1 Set-up as used in (Johnston 2016) showing the target used for focusing.
A printout target was used to focus both the laser and camera, see Figure 3.1 (Johnston 2016). Initially a candle was used as the soot source and positioned in line with the target on top of a matt black surface to prevent specular reflections.
A series of background control photos were taken at 1000fps to clearly distinguish signals caused by LII. The first images taken were at different laser energies, images at a range of shutter speeds then followed (250fps, 1000fps and 5000fps).
The position of the candle relative to the laser was increased with the aim of allowing the excitation optics to form a consistent sheet. The experiment was performed again at half and full laser power, with the additional camera settings of 50fps and 500fps.
Several other techniques were used in attempts to obtain a signal:
No LII signals were received despite the various attempts made, this was attributed to several possible reasons. It was suggested the Litron did not have enough power, this was later dismissed by Johnston (2016) since the Litron had the capacity to run at the fluence level desired for LII.
Another explanation Johnston (2016) suggested was that the software used could not take single frames of images. The image intensifier was used to rectify this via temporally gating the image, yet still no signal was received. The HiSense MKII camera running a PLIF set-up at the GTRC uses software that can capture individual frames at selected times.
Furthermore, Johnston (2016) suggested that the soot source was to blame despite attempts to rectify the issue. The use of Cardiff University’s graphite particle generator was suggested as a consistent strong sooting source.
The detection filter used may not have been sufficiently effective as to block out the orange emissions from the flame. However, the most likely explanation behind the lack of signal was the shortfall in camera intensity directed at the point where the laser interacts with the soot (Johnston 2016).
Following the findings of Johnston (2016), the fundamental aim for this project was to implement a functioning LII system at the Cardiff University GTRC. The purpose of the GTRC is to simulate gas turbine combustion for research purposes. A functioning LII system at the GTRC would generate additional future opportunities for the study of soot volume fraction formation as well as particle size for a range of alternative fuel types.
The goal was firstly to explore whether the technology currently being used for PLIF studies could be altered for the study of LII. If the PLIF set-up could not be modified, it would be a case of exploring another approach with the implementation of a separate detection set-up.
There are three fundamental aspects of a non-intrusive in-exhaust stream LII System; the soot source, the laser and the detection optics. The following section discusses the thought rationale in specifying each of these, a schematic of the final experiment layout is displayed in Figure 4.1.

Figure 4.1 Schematic diagram showing LII experiment set-up at the GTRC.
The capability of the laser in an LII set-up is the single most important aspect. If there is not enough power to cause the soot particles to incandesce then the experiment will not be successful. As mentioned in Section 2.9, the ideal laser output wavelength is 1064nm as it removes the possibility of unwanted LIF signals interfering with the detected signal. However, studies comparing LII at the fundamental and secondary harmonics (Schulz et al. 2006, Therssen et al. 2007, Wainner and Seitzman 1999) have shown a laser operating at 532nm with a notch filter attached to the detection system, is still suitable to perform LII effectively.
The laser most suitable for this demonstration application owned by Cardiff School of Engineering is a Litron Nano S PIV, pictured in Figure 4.2. The Litron is a Class 4 PIV laser with a maximum output energy of 30mJ, beam diameter of 4mm, pulse duration of 7ns and a repetition rate of up to 20Hz. According to these parameters, the laser should in theory be powerful enough to cause the soot particles to incandesce (optimum fluence level for 532 nm excitation is 0.2 J/cm2 – Section 2.9.2):
Fluence Jcm2=Energy per pulse (J)Beam area (cm2)= 0.03π0.424=0.24 Jcm2

Figure 4.2 Picture showing Litron Nano S PIV laser set-up in position at the GTRC
A variable optical attenuator is fitted to the laser to control the output energy. This output energy is further attenuated via the use of an extra-cavity polariser and half wave plate, thus maintaining beam quality and divergence. The laser system adopts two pulsed and Q-switched Nd:YAG lasers producing infrared 1064nm light, the beams are then combined and aligned co-axially using polarising beam combination optics. For the primary use of this laser namely PIV, the 1064nm light is frequency doubled to visible 532nm light using a Harmonic Generation Assembly (HGA), 532nm separation optics then follow in the form of dichroic mirrors before exiting the laser head. Any additional 1064nm light is directed towards a beam dump in line with the HGA. A cutaway of the laser head is displayed in Figure 4.3, the path of the 1064nm light is shown in red and the 532nm path in green.

Figure 4.3 Cutaway of Litron Nano S PIV laser displaying optics and light path. (Origin of original photo: Litron Lasers Ltd. 2013).
Since a laser producing 1064nm is desirable for LII purposes, the possibility of modifying the Litron laser as to not emit 532nm light was explored. By analysing the internal optics, it was thought that by removing the HGA unit, the 1064nm light could be directed along the path which the 532nm light currently occupies. However, it was discovered that the dichroic mirrors currently in place would not be able to cope with 1064nm light and would ultimately burn out. This meant that to generate laser light at the fundamental harmonic the laser would require additional parts to be purchased to do so. Ultimately the laser was run in standard 532nm configuration producing a 1D Gaussian laser profile, a support was fabricated and then the laser positioned as shown in Figure 4.2.

Figure 4.4 Picture showing optical combustion measurement section at GTRC.
The large fuel mixing facility at the GTRC is central to the development of renewable fuels as the world moves away from conventional sources of energy. In use at the GTRC is a gas turbine relevant combustor. The principal advantage of this device is that the section housing the combustion chamber has optical access with multiple windows, thus enabling various optical measurement techniques to take place. This section is displayed in Figure 4.4.
 Currently PLIF experiments are performed at this optical combustion section using a “Spectra Physics Lab 170” Nd:YAG laser coupled to a “Quantel 90” tunable dye laser. Positioned directly above the combustion chamber is a “Dantec HiSense MKII” CCD camera to perform PLIF. These experiments are highly sensitive to changes in set-up and would require extensive re-calibration if either the laser or camera is modified. As a result, if the LII experiment was to utilise either of these, efforts must be made to minimise disruption.
Currently PLIF experiments are performed at this optical combustion section using a “Spectra Physics Lab 170” Nd:YAG laser coupled to a “Quantel 90” tunable dye laser. Positioned directly above the combustion chamber is a “Dantec HiSense MKII” CCD camera to perform PLIF. These experiments are highly sensitive to changes in set-up and would require extensive re-calibration if either the laser or camera is modified. As a result, if the LII experiment was to utilise either of these, efforts must be made to minimise disruption.
Figure 4.5 Picture displaying exhaust section showing laser in position and point of access for camera.
Currently the Quantel laser dumps a large proportion of 1064nm light. Previously Johnston (2016) explored utilising this light for use with LII, however as to not disrupt the PLIF set-up it was decided to use the Litron Nano S PIV. The use of the HiSense MKII CCD camera was however explored. It was thought that if the laser could be positioned perpendicular to the camera with the beam entering the optical window seen in Figure 4.4 there would be minimum disruption to the PLIF set-up when operating LII. Yet there was concern that the 532nm light would damage the intensifier attached to the camera meaning it could not be used without disrupting the PLIF set-up. It was therefore decided to perform the LII experiment further downstream to allow the use of an additional camera.
The location decided upon was a section of the exhaust which had been previously adapted to facilitate optical access from each orientation, allowing the CCD camera to be positioned directly above perpendicularly to the laser, thus being an optimum set-up for in-situ LII. This optical section of exhaust is shown in Figure 4.5, indeed note the positioning of the laser with the optical access hole for the camera adjacent to it.
An LII photodetector set-up typically consists of either a single element detector such as a photomultiplier tube (PMT) or in the case of this experiment, a CCD camera. The camera collects a series of images along the trajectory of the laser which if there is an incandescent signature appear as LII ‘spots’. The intensity of the LII spot image can be directly linked to the mean soot volume fraction providing correct calibration has taken place (however this was not within the scope of this study, which was a proof of concept exercise). Primary particle size is then calculated from the time decay of the LII signal.
The device chosen for the experiment, which was loaned from Rolls-Royce aero was an Andor iStar ICCD camera. ICCD cameras are simply CCD cameras with an integrated gated image intensifier, this is used to aid in gating the image with the pulse generated by the laser, acting as an enhanced optical shutter. Also, helping to block out background light that may be present and interfering in the testing environment.
An essential part of obtaining quantitative information from an LII signal is how the light is filtered through the detector lens. Present on the day of the experiment were two narrowband LII filters (430nm and 480nm) which transmit light from specific wavelengths to the photodetector, any other forms of light are blocked. However, a notch filter is preferred which blocks out interference from the incident beam allowing the best chance of a signal being picked up by the detector. A notch filter rated at 532nm was not available on the day of the experiment (however has subsequently been specified – Section 5.2) and after concluding that using a narrowband filter would limit the findings, it was decided to run without a filter on the camera to maximise the possibility of receiving an LII signal.
To measure and quantify soot particles generated from combustion, the ability to produce particles in a way that is both reliable and repeatable is essential. Although the gas turbine combustor at the GTRC is operated for various research purposes, the use of it was not required for verifying if the LII system would function as desired. If the experiment proved successful then there would be scope to utilise the LII system with a range of fuel types. Instead a graphite particle generator was used to simulate soot production from the gas turbine, the Palas GfG 1000 is pictured in Figure 4.6.

Figure 4.6 Picture showing Palas GfG 1000 graphite particle generator.
The GfG 1000 produces test aerosols similar to soot, generated by a spark between two graphite electrodes placed close together with a high potential difference applied between the two. The electrodes are situated in a flow of argon gas, the graphite evaporates as the spark is produced and then re-condenses to form nano particles in the gas which then form agglomerates during the flow path. The potential difference, distance between electrodes and energy converted by each spark, remains constant resulting in a consistent particle distribution (Filter Integrity Ltd. 2010).
The argon gas is generated at a volume flowrate of 1.5 l/min and at a primary pressure of 4 bar. Particle concentration can be as much as 107 particles/cm3 with a primary particle size of 3 nm (Filter Integrity Ltd. 2010). The flow of particles is then fed through a tube into and through the exhaust stream (white tube in foreground of Figure 4.7), by feeding it all the way through to the point of detection it ensures the flow of particles meet the laser light as a point release offset at 90.

Figure 4.7 Picture showing graphite particle entry point.
The experiment at the GTRC was set-up as described in Section 4.0, using the Litron Nano S PIV laser, Andor iStar ICCD camera and the Palas GfG 1000 particle generator. Supports had to be fabricated specifically for both the laser and the camera; measurements were taken then once attached and in position it was imperative to ensure that both were perfectly aligned. Naturally if the camera is not aligned and focused with the laser then no signal will be received. The ICCD camera was linked with Andor’s software to allow the measurements to take place, it was then important to focus the camera to ensure that it was at the correct resolution to record an LII signal. A steel rule was positioned along the mid-plane of the pipe with the camera then adjusted accordingly, this also provides a scale for the camera in pixels/mm.
Whilst the final preparations were being made, the pump used to heat the laser was turned on to ensure that it would be able to perform the experiment at maximum capacity. The GTRC has a door interlock system for the test cell which must be activated before the laser is switched on, if any door into the test cell is opened the laser automatically shuts down. Any individuals remaining in the test cell must then wear the appropriate laser goggles to prevent any possible eye damage.
Once the door interlock had been activated the laser start-up process was initiated. The laser itself has two interlock systems; a ‘simmer’ and ‘water flow’ interlock. If a water flow has been established from the pump then the respective interlock will be extinguished. The maximum energy setting and pulse duration of 7ns was then selected allowing the laser to be turned on. Once the laser is on there is a slight delay before the simmer interlock is established, if successful the charger will enable and the interlock will extinguish. Finally, the emission indicators illuminate notifying the user that the laser is ready to be activated.
The ICCD camera was operating in ‘Real Time’ mode whereby the scanning is controlled by an internal clock which is not synced to the laser in any way, it was however gated slightly longer than the 7ns laser pulse to allow for temporal inaccuracies. The particle generator was then switched on with an argon flow rate of 1.5 litres/min before activating the laser.
The experiment was run twice. In the first run, the camera was picking up too much background light coming from the test cell, this was rectified by repeating the process with the test cell lights switched off.
 5.2 Results and Discussion
5.2 Results and DiscussionFigure 5.1 Image displaying LII ‘spot’ signal received from experiment.
Positively, the experiment proved successful and an LII signal was received. An image of the signal detected from the second run of the experiment when the test cell lights were turned off is shown in Figure 5.1. This image depicts two separate LII signals, this is due to the scanning of the ICCD detector running in the ‘Real Time’ mode. In reality there is only one signal and if triggering information is obtained for the laser it would in theory be possible to sync it to the detector and mitigate the issue.
Both the laser beam and the particle stream emerging from a tube are cylindrical. As stated previously the laser has a 1D Gaussian profile while the particle stream as it exits the nozzle resembles a Top Hat profile. When viewed from the positon of the camera above, the two interact at right angles resulting in the most intense aspect of the LII image along the line where the centres overlap, the intensity will then drop off as it travels further from that point. It is this change in intensity which yields the approximately circular image; previously referred to as LII ‘spots’.
The experiment proved that the proposed LII system was achieving an LII signal, yet currently it would not be possible to obtain any quantitative information about the nature of the generated particles; the lack of notch filter to reject scattered 532nm light is the main reason behind this. It is also worth noting that that although the equipment was aligned as meticulously as possible (under the time of the set-up period), the extent of the overlap between the components is not known. If an extensive alignment process was undertaken then the uncertainties based on misalignment could be reduced. Nevertheless, after confirming the technology works as desired, the ability to obtain quantitative results is something which could be optimised in the future.
To take the design forward and obtain quantitative information there are multiple options which could be explored, the first of which is purchasing a 532nm notch filter which can be attached to the Andor iStar detector. Alternatively, the Litron Nano S PIV could be upgraded to operate in 1064nm configuration, thereby removing the need for a filter as the intensifier built into the detector is not sensitive at that wavelength. Another option would be to use an alternative laser running at 1064nm, this could prove favourable if an upgrade to the Litron was either not possible or not financially viable. The benefits of running the experiment using a 1064nm over a 532nm laser are discussed in Section 2.9.5. Although it is entirely possible to perform LII at 532nm, the necessity for additional filters complicates matters. Running LII at the fundamental harmonic of 1064nm is favoured.
Currently at Cardiff University there is an out of service Nd:YAG laser, the Spectron Laser Systems SL456G capable of emitting laser pulses at 1064nm. It has a pulse duration of 6 ns, repetition rate of 10 Hz and a maximum pulse energy of 1100 mJ per pulse, thus making it ideally suited for LII. However, it is a much larger unit than the Litron laser currently being used which could prove problematic, especially as a key motive behind the design of the LII system was an element of portability.
The alternative to using a different laser is upgrading the current Litron Nano S PIV to operate at the fundamental harmonic. Litron Lasers currently offer two additional head options to the standard 532nm output, these allow either a double pulsed ultra-violet output of 355 and 266 nm, or a double pulsed mixed visible and ultra-violet output of 532, 355 and 266 nm. Although suitable for LIF purposes, neither is applicable for LII.
If it was achievable, deemed safe and Litron Lasers could provide appropriate training, then the internal excitation optics of the laser could be modified to output at 1064 nm. There is thought that the HGA unit (see Figure 4.2) could be relocated along with the first dichroic mirror which follows it. If the 1064 nm beam dump was then removed this would allow the beam to exit through the vacant port at 1064 nm having not had its frequency doubled.
Alternatively, if neither options to run at 1064nm are possible then a notch filter could be purchased to run an enhanced version of the 532nm set-up. At a cost of £641 (excluding VAT) Semrock produce a “532 nm StopLine® single-notch filter” with a diameter of 25mm to fit the lens of the Andor iStar. The transmission spectrum for this filter, reference NF01-532U-25, is displayed in Figure 5.2. The extremely narrow drop in transmission allows filtration of the 532 nm laser radiation.

Figure 5.2 Transmission spectrum for 532 nm StopLine® single-notch filter (Laser 2000).
Having proved successfully that a basic LII system will function at the GTRC, any future work should target obtaining quantitative information on particulates using LII. The set-up of the experiment at the GTRC should be optimised prior to any further investigations involving soot volume fraction and particle sizes.
The Litron Nano S PIV should be modified to emit light at 1064 nm via removal of the HGA unit and the respective dichroic mirror which follows. If the laser is running at the fundamental harmonic then a notch filter will not be required due to lack of sensitivity in the infra-red region experienced by the Andor iStar. Assuming triggering information has been obtained, the laser should be synced to the detector to ensure that a single image is received. Great attention should also be paid to the overlap between the laser beam and detector, if both are not perfectly aligned then there is little chance of obtaining any quantitative information.
To deduce a value for relative soot volume fraction using LII, a form of calibration is essential to compare the signal recorded by the photodetector to the concentration of soot being generated. Traditionally LII results are compared to a system with a soot volume fraction which has been recorded via an additional measurement technique which is assumed to provide accurate results (Smallwood 2009).
As LII has continued to provide quantitative information on soot particulates, various calibration methods have been performed to accommodate individual scenarios. These range from the use of a 40kW kerosene burner in custom combustion set-up (Black et al. 2008), to a new, less conventional technique called auto-compensating LII (Snelling et al. 2005). This technique does not require additional soot measurement tests but instead the use of a calibrated light source to provide illumination to the same photodetector equipment being used for LII. Extensive research should be undertaken as to what method is best suited to optimising the LII system at the GTRC.
The key step is establishing a fully calibrated and functioning LII system at the GTRC, once that has been achieved there are several opportunities for future uses. The system is relatively compact meaning there is potential to transport it for uses not associated with the combustion chamber at the GTRC. Yet, at the time of writing there is an extractive LII experiment operating in the same optical section of exhaust at the GTRC. It would be an attractive proposition to confirm the theory that an extractive LII system is less accurate than an in-situ LII system due to the particulate losses through transport of the sample. Furthermore, the fuel mixing facility at the GTRC provides the perfect opportunity to analyse the sooting characteristics for a range of different fuel types, congruent with the fundamental drive to reduce particulate emissions which are a direct consequence of combustion processes.
To conclude, acknowledging the limitations of this short experiment, this single researcher study succeeded in establishing the principles of LII. This success can be built upon via further development of the system to stimulate future research opportunities. Through careful alignment, modifications to the laser excitation optics and calibration of the system, there is clear potential in utilising this non-intrusive LII process for the quantitative measurement of soot. This area of research is of global importance to the health of individuals and the ever-increasing threat of climate change.
Automobile Association Developments Limited (GB) 2015. Euro Emissions Standards – Limits to improve air quality and health [Online]. Available at: https://www.theaa.com/driving-advice/fuels-environment/euro-emissions-standards [Accessed: 6 March 2017].
Axelsson, B. et al. 2000. Laser-induced incandescence for soot particle size measurements in premixed flat flames. Applied Optics 39(21), pp. 3683-3690.
Bengtsson, P.E. and Alden, M. 1995. Soot-visualization strategies using laser techniques – Laser-induced fluorescence in C2 from laser-vaporized soot and laser-induced soot incandescence. Applied Physics B 60(2), pp. 51-59.
Black, J.D. et al. 2008. In-Situ Laser-Induced Incandescence of Soot in Large Civil Aero-engine Exhausts. 26th AIAA Aerodynamic Measurement Technology and Ground Testing Conference. CNRS, June 2008. France.
Bladh, H. and Bengtsson, P. 2004. Characteristics of Laser-Induced Incandescence from Soot in Studies of a Time-Dependent Heat-and Mass-Transfer Model. Applied Physics B: Lasers and Optics 78(2), pp. 241-248.
Bockhorn, H. ed. 1994. Soot Formation in Combustion. Berlin: Springer-Verlag.
Bohren, C.F. and Huffman, D.R. 1983. Absorption and Scattering of Light by Small Particles. New York: John Wiley & Sons.
Bredin, A. et al. 2011. Thermogravimetric analysis of carbon black and engine soot – Towards a more robust oil analysis method. Tribology International 44(12), pp. 1642-1650.
Case, M.K. and Hofeldt, D.L. 1996. Soot Mass Concentration Measurements in Diesel Engine Exhaust Using Laser Induced Incandescence. Aerosol Science and Technology 25(1), pp. 46-60.
Dalzell, W.H. and Sarofim, A.F. 1969. Optical constants of soot and their application to heat flux calculations. Journal of Heat Transfer 91(1), pp. 100-104.
European Commission 2016. Environment – Air Quality Standards [Online]. Available at: http://ec.europa.eu/environment/air/quality/standards.htm [Accessed: 6 March 2017].
Filter Integrity Ltd. 2010. Nano Particle Aerosol Generators – GFG Series [Online]. Available at: http://www.filterintegrity.com/PTAS/PandS/Products/SolidPartGen/Spark/GFG.html [Accessed: 27 March 2017].
Grisch, F. and Orain, M. 2009. Role of Planar Laser-Induced Fluorescence in Combustion Research. Aerospace Lab Journal [Online] 1. Available at: http://www.aerospacelab-journal.org/sites/www.aerospacelab-journal.org/files/Al1-11_0.pdf [Accessed: 19 March 2017].
Haynes, B.S. and Wagner, H.Gg. 1981. Soot Formation. Progress in Energy and Combustion Science 7(4), pp. 229-273.
Johnson, M. 2015. Rolls Royce – Derby.
Johnston, H. 2016. Design of a Non-Intrusive Laser System for the Measurement of Combustion-Derived Soot. Cardiff University.
Keefe, Z. 2013. Industrial Hygiene – What is Soot and Why is it Dangerous? Cashins & Associates Blog [Online]. Available at: http://blog.cashins.com/blog-0/bid/191511/Industrial-Hygiene-What-is-Soot-and-Why-is-it-Dangerous [Accessed: 21 March 2017].
Kempthorne, T.H. 2010. Development of a Laser-Induced Incandescence System. MSc Thesis, University of Toronto.
Kennedy, I.M. 2007. The health effects of combustion-generated aerosols. Proceedings of the Combustion Institute 31(2), pp. 2757-2770.
Kholghy M.R. 2012. The Evolution of Soot Morphology in Laminar Co-Flow Diffusion Flames of the surrogates for Jet A-1 and a Synthetic Kerosene. MSc Thesis, University of Toronto.
Lapuerta, M. et al. 2006. Thermogravimetric analysis of diesel particulate matter. Measurement Science and Technology 18(3), pp. 650-658.
Laser 2000 Advanced Solutions for Photonics. 2017. Notch Filters – NF01-532U-25 [Online]. Available at: http://www.laser2000.co.uk/semrock_filter.php?code=NF01-532U-25 [Accessed: 1 April 2017].
Litron Lasers Ltd. 2013. Laser System User Manual.
Mansurov, Z.A. 2005. Soot formation in combustion processes. Combustion, Explosion and Shock Waves 41(6), pp. 727-744.
Marinov, N.M. et al. 1999. The Formation of Aromatics and PAH’s in Laminar Flames. Joint Meeting of the British, German and French Sections of the Combustion Institute. Nancy, 18-21 May 1999. France.
Melton, L.A. 1984. Soot Diagnostics Based on Laser Heating. Applied Optics 23(13), pp. 2201-2207.
National Cancer Institute (US) 2015. About Cancer – Cancer-Causing Substances [Online]. Available at: https://www.cancer.gov/about-cancer/causes-prevention/risk/substances/soot [Accessed: 21 March 2017].
Nepf, H. 2002. Laser Induced Fluorescence (LIF) – Massachusetts Institute of Technology [Online]. Available at: http://web.mit.edu/fluids-modules/www/exper_techniques/3.Laser_Induced_Fluorescenc.pdf [Accessed: 19 March 2017].
Richter, H. and Howard, J.B. 2000. Formation of polycyclic aromatic hydrocarbons and their growth to soot – a review of chemical reaction pathways. Progress in Energy Combustion Science 26(1), pp. 565-608.
Richter, H. et al. 2004. Detailed modelling of pah and soot formation in laminar preliminary mixture benzene/oxygen/argon at low pressure flame. Proceedings of the Combustion Institute 30(1), pp. 1397-1405.
Rohlfing, E.A. 1988. Optical emission studies of atomic, molecular, and particulate carbon produced from a laser vaporization cluster source. The Journal of Chemical Physics 89(15), pp. 6103.
Santoro, R. et al. 1983. Soot particle measurement in diffusion flames. Combustion and Flame 54(1), pp. 203-218.
Schulz, C. et al. 2006. Laser-induced incandescence: Recent trends and current questions. Applied Physics B 83(3), pp. 333-354.
Smallwood, G. and Schulz, C. 2005. LII Science – Target Flames [Online]. Available at: http://liiscience.org/g%C3%BClder_burner/ [Accessed: 2 December 2016].
Smallwood, G.J. 2009. A Critique of Laser-Induced Incandescence for the Measurement of Soot. PhD Thesis, Cranfield University.
Snelling, D.R. et al. 1997. Development and Application of Laser-Induced Incandescence (LII) as a Diagnostic for Soot Particulate Measurements. AGARD Conference proceedings 598, pp. 23.1-23.9.
Snelling, D.R. et al. 2000. Evaluation of the Nanoscale Heat and Mass Transfer Model of the Laser-Induced Incandescence: Prediction of the Excitation Intensity. Thirty Fourth National Heat Transfer Conference.
Snelling, D.R. et al. 2005. A calibration-independent LII technique for soot measurement by detecting absolute light intensity. Applied Optics 44(31), pp. 6773-6785.
Srinivasarao, R. 2013. Particulate matter from refinery flares and Health Effects of Soot. International Journal of Information Technology and Business Management 6(1), pp. 78-83.
The Environment Literacy Council 2015. Air & Climate – Black Carbon (Soot) [Online]. Available at: https://enviroliteracy.org/air-climate-weather/climate/black-carbon-soot/ [Accessed: 21 March 2017].
Therssen, E. et al. 2007. Determination of the ratio of soot refractive index function E(m) at the two wavelengths 532 and 1064 nm by laser induced incandescence. Applied Physics B 89(2), pp. 417-427.
Troyer, D. 1999. Get ready for more Soot in Engine Oil [Online] Available at: http://machinerylubrication.com/Read/51/soot-oil-engine [Accessed: 22 March 2017].
Turns S.R. 2006. An Introduction to Combustion: Concepts and Applications. New York: McGraw-Hill Education.
US EPA 2012. Black Carbon Report to Congress – Chapter 2, Black Carbon and Its Effects on Climate [Online]. Available at: https://www3.epa.gov/airquality/blackcarbon/2012report/ Chapter2.pdf [Accessed: 21 March 2017].
van Setten et al. 2001. Science and technology of catalytic diesel particulate filters. Catalytic Reviews 43(4), pp. 489-564.
Vander Wal, R.L. 1995. The effects of rapid heating of soot: Implications when using laser-induced incandescence for soot diagnostics. Combustion and Flame 102(1), pp. 200-204.
Vander Wal, R.L. and Weiland, K.J. 1994. Laser-Induced Incandescence: Development and Characterization Towards a Measurement of Soot-Volume Fraction. Applied Physics B 59(4), pp. 445-452.
Wainner, R.T. and Seitzman J.M. 1999. Soot diagnostics using laser-induced incandescence in flames and exhaust flows. American Institute of Aeronautics and Astronautics.
Wang, H. 2011. Formation of nascent soot and other condensed-phase materials in flames. Proceedings of the Combustion Institute 33(1), pp. 41-67.
Weeks, R.W. and Duley W.W. 1974. Aerosol-particle sizes from light emission during excitation by TEA CO2 laser pulses. Applied Physics 45(10), pp. 4661-4662.
Weidman, J. and Marshall, S. 2010. Soot Pollution 101 – What You Need to Know and How You Can Help Prevent It. Centre for American Progress [Online]. Available at: https://www.americanprogress.org/issues/green/news/2012/08/10/12007/soot-pollution-101/ [Accessed: 21 March 2017].
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more