What is radiation
Radiation is the emission of energy in the form of rays or waves and is classified into two categories; ionizing and non-ionizing. This classification is dependent on the amount of energy it emits when knocking off electrons from its target. Ionizing radiation involves unstable atoms giving off energy in order to reach a stable state. This presents a health threat to humans since it changes the basic makeup of atoms in cells. These ionizing radiations could interact directly with the DNA thus disrupting the molecular structure causing cell damage such as base pairing change, breakage and gene mutation causing targeted effects. At high LET radiations such as alpha particles, neutrons and high radiation doses, these damages could induce carcinogenesis (Goodhead, 1994). However, it can also cause indirect damage where oxygen species are formed from ionised water and other organic molecules interacting to produce free radicals which causes non-targeted effects in the genome. These free radicals are very reactive since they have an unpaired electron in the structure thus reacting with a DNA molecule to cause structural damage (Hu et al., 2012). The number of free radicals produced by the ionizing radiation depends on the total radiation dosage. Whereas non-ionizing radiation is a relatively low energy radiation that is unable to ionize atoms or molecules. These types of radiation include power lines, microwaves, lasers and radio waves and are considered harmful only where there is overexposure to the radiations.
Sources of radiation
The main sources of radiation exposure are natural radiation and artificial radiation. The exposure to natural radiation could come from three different sources as seen in figure 1; first, cosmic radiation which typically carries beta and gamma radiation. The charged particles of radiation are formed through the interaction of the sun and starts with the earth’s atmosphere and magnetic field. Second, terrestrial radiation where radioactive atoms formed from uranium and thorium are found in soil, water, vegetation and therefore consumed in food and water. Lastly, internal radiation where radioactive materials such as potassium-40, carbon-14 and lead-210 are found in human bodies since birth and about 30,000 atoms decay in humans’ lungs each hour. On the other hand, artificial radiation exposure is divided into two groups; the occupationally exposed individuals, who are individuals whose mean of work involves some exposure of radiation such as X-ray technicians, nuclear medicine technicians and flight crew. However, members of the public could also get radiation exposure from man-made radiation sources such as tobacco, televisions, combustible fuels and radiation therapy (USNRC Technical Training Center, 2017).

Figure 1: The chart illustrates the average dosage of the American population where 82% of the ionizing radiation exposure to the public comes from natural sources with radon holding the largest percentage of ionizing radiation exposure at 55%. Whereas the remaining 18% is accounted for man-made sources (USNRC Technical Training Center, 2017).
Radiation therapy
The use of ionizing radiation in clinical treatment of cancer has become an essential course of treatment alongside surgery and systemic therapy, as more than 60% of cancer patients receive radiotherapy (Orth et al., 2014). Radiotherapy has proven to control tumour growth rate and prolong patient’s survival rate. In lung cancer, radiotherapy is used in the treatment of early forms of bronchial carcinoma since it gives high rate of local control thus avoiding invasion into surrounding tissues. For advanced stages, it can be used in a neoadjuvant, adjuvant, or definitive manner as well as for palliation, respectively (Timmerman et al., 2010). As ionizing radiation is effective in killing eukaryotic cells, this raises the strategies in which it is used in tumour treatments. Thus, recent advances such as IMRT allows in limiting and controlling the dose of radiation that is exposed to the normal tissues whilst using a prescribed dose on the tumour cells. This is not to say that normal cells are not exposed to any ionizing radiation, in fact, they still are exposed to low doses which could increase the risk of radiation induced secondary cancers (Hall, 2009). The dose of ionizing radiation depends on the type and stage of cancer that is being treated, in curative cases, epithelial tumour treatment ranges from 60 to 80 Gy, whereas lymphomas are treated with 20 to 40 Gy. Furthermore, adjuvant doses range between 45 to 60 Gy in 1.8 to 2 Gy fractions.
Targeted and non-targeted effects of radiation
The linear-non-threshold (LNT) suggests that low dose radiation exposure results in the induction of oncogenesis and as radiation exposure increases, the risk of carcinogenesis increases subsequently in a direct linear proportion regardless of the radiation dose rate.
The effects of radiation are grouped into two distinct categories: deterministic effects and stochastic effects. Deterministic effects are the immediate effects of radiation where cells die at low levels of radiation exposure. Whereas stochastic effects are a chronic continuous long-term exposure to radiation where there is a delay in the observed health effects (Kadhim, Moore and Goodwin, 2004). Nonetheless, as the threshold dose of exposure to radiation increases, the severity of the injury increases with the dose. These continuous exposures to radiation can be harmful since it could lead to the development of carcinogenesis, changes in somatic cells, genetic mutations, cataracts and benign tumours (Burtt, Thompson and Lafrenie, 2016).
The targeted effects of radiation exposure have been studied for many years implying that the effects of radiation such as chromosomal aberrations and cell death were a direct result of energy deposition from ionizing radiation that effected cells with the assumption that DNA is the primary target demonstrated in figure 2. The DNA damage responses are regulated by two conserved protein kinases, called Ataxia telangiectasia mutated (ATM) and Ataxia telangiectasia and Rad3 related (ATR) (Smith et al., 2010). Mre11-Rad50-Nbs1 (MRN) complex from DSBs recruit ATM where it phosphorylates histone H2 variant thus creating a platform for other DDR factors. ATM resects the broken DNA strands where then the ssDNA repair intermediates activate ATR kinase by phosphorylating CHK1 and CHK2 kinases. Therefore, ATR and ATM trigger signalling pathways in which they initiate arrest within cell cycle progression and DNA damage repair by phosphorylating tumour suppressor protein p53 where it disassociates from MDM2 thus stabilised thus able to regulate cell cycle arrest, DNA damage repair, and the induction of cell death or senescence by inducing or repressing the expression of several target genes that encode for factors involved in these processes (Meek, 2009). Furthermore, they can induce cellular senescence or cell death in excessive DNA damage (Jackson and Bartek, 2009).
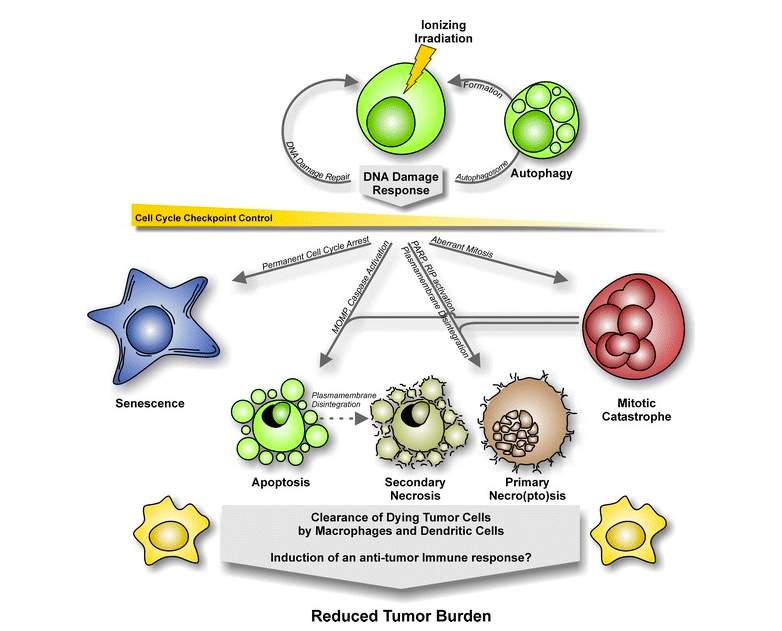
Figure 2: Mechanisms of cell death triggered by ionizing radiation (Orth et al., 2014)
Nonetheless, in the early 1990s, evidence of non-targeted effects were discussed and reviewed, which resulted with NTE being characterised by cellular responses that occur in the progeny of irradiated somatic/germ cells known as genomic instability and cells influenced by energy deposition in neighbouring irradiated cells as a result of intercellular signalling called the bystander effect (Kadhim and Hill, 2015) .

Figure 4: illustration of models representing targeted and non-targeted effects of radiation exposure on cells (Kadhim and Hill, 2015).
Non-targeted effects of radiation
Radiation induced bystander effect
Bystander effect is biological outcomes observed in non-irradiated cells consequently occurring from an exposure of ionizing radiation to other cells within a population (Desouky, Ding and Zhou, 2015). Radiation induced bystander effects occurs as a result of cellular responses due to cell-to-cell communication or though the release of soluble factors within the cell’s microenvironment (Kadhim et al., 2013; Morgan and Sowa, 2015). Therefore, the genetic damage caused to the DNA is not only directed to the irradiated cells but rather to the non-irradiated cells as well. The two main pathways of cell signalling involved in the radiation induced bystander effect could either be short range gap junction intercellular communication (GJIC) or long range distant cell signalling intercellular communication (DSIC) (Kadhim and Hill, 2015). Gap junction channels depend on the transfer of small molecular weight molecules reactive oxygen species (ROS) and reactive nitrogen species (RNS) from irradiated to non-irradiated cells. Whereas DSIC involves transmissible signals produced from irradiated cells such as cytokines calcium fluxes and exosomes (Kadhim and Hill, 2015).
Short range intercellular communication of bystander effect
Short range bystander effect is demonstrated through the production of reactive oxygen species and reactive nitrogen species. It is known that the exposure of ionizing radiation causes the initial formation of water radiolysis that results in the immediate production of hydrated electron, hydroperoxyl radical, hydroxyl radical, hydrogen radical and hydrogen peroxide. The reactive oxygen species associated with biological effects such as cell proliferation to carcinogenesis are superoxide radical and alkyl hydroperoxide (Yamamori et al., 2012). Further studies have indicated that once cells have been exposed to ionizing radiation, the production of the intracellular ROS is upregulated several hours post exposure signifying a secondary biological source responsible for the production of ROS; that is the mitochondria. A study concluded that functionally active mitochondria is responsible for ROS production due the increased mitochondrial membrane potential and enhanced mitochondrial respiration seen in their results. Furthermore, a study conducted by Chen and colleagues proposes the initiation of the intercellular bystander signalling seen from irradiated cells uses a mitochondrial-dependent pathway observed in the early stages of radiation exposure, which eventually stimulate the over-expression of ROS levels in cells (Chen et al., 2009). Therefore, leading to an increase in double stranded breaks, chromosomal aberration and delayed genomic instability in bystander cells.
Nonetheless, the production of free radicals in the mitochondria can also be stimulated by NADPH Oxidase, expressed by protein kinase c and p38 (Yamamori et al., 2000). NADPH oxidase generates a superoxide anion by transferring electrons from cytosolic NADPH across the cell membrane to the extracellular molecular oxygen, therefore, upregulating the superoxide formation due to more electron release from the mitochondria (Najafi et al., 2014). It has been reported that the suppression of NADPH oxidase enzyme post radiation exposure reduces the biological effects on hematopoietic cells (Pazhanisamy et al., 2011).
Nitric Oxide (NO) is an essential signalling molecule associated with the immune, cardiovascular and nervous system due to its stability and hydrophobic properties. These properties allow it to diffuse through the cytoplasm and plasma membranes thus creating a signalling pathway in bystander cells (Yakovlev, 2015). A study showed that X-ray irradiation activates inducible NOS expression 3 hours after exposure continuing to increase its activity over a 24-hour post irradiation. The overexpression of NO stimulates proliferation and shorten the cell cycle in bystander cells thus the damaged DSBs will not be repaired which will eventually generate genomic instability (Yakovlev, 2015).
long range distant cell signalling intercellular communication of bystander effect
Long range distant cell signalling involves the excretion of cytokinesis; the TGF-β1 pathways get activated once the cells are exposed to ionizing radiation in vitro, releasing TGF-β1 signalling molecules into the medium which diffuse to the non-irradiated cells and thus activating the pathway in them exhibited in figure 3. This activation is then responsible for the regulation of miR-21 expression in the bystander cells, elevated levels of miR-21 induces oxidative stress leading to damage in the DNA (Jiang et al., 2014).
Exosomes are small molecules released by cells in the extracellular microenvironment and body fluids that play a role in the cell-cell communication. When cells are exposed to ionizing radiation, exosomes diffuse from the irradiated cells and travel in the extracellular medium to be taken up by non-irradiated cells. Studies showed evidence of miR-21 shuttling from the irradiated cells into the cytoplasm of the bystander cells through exosomes (Xu et al., 2015). Further studies also confirmed the release of exosomes in a 2Gy X-ray irradiated cells in MCF-7 breast cancer cells which also showed elevated levels of DNA damage (Kadhim and Hill, 2015).

Figure 3: A model presenting the damage that occurs to the DNA via cytokines in radiation-induced bystander effects (Jiang et al., 2014).
Radiation induced genomic instability
Genomic instability refers to the delayed effects of radiation that occurs in the progeny of cells many generations after the event of radiation. These effects include delayed gene mutations, gene amplifications, chromosomal damage, micronucleus formation and de novo chromosomal aberrations as well as transiting normal cells to oncogenesis (Kadhim and Hill, 2015). Genomic instability is observed over many cell generations where chromosomal damage was detected in later cell divisions that affected the whole cellular genome. A study showed that in a mouse strain the development of a genetically predisposed gastroschisis and the induction of genomic instability measured by chromosomal aberrations showed that mice that develop the malformation after irradiation during the zygote stage also develop other skeletal malformations. Furthermore, the frequency of gastroschisis and other developmental defects are significantly increased in the next mouse generation although no further radiation exposure took place (Streffer, 2000). These radiation-induced damages could have originated from direct interaction of reactive oxygen species with the DNA or effects of ROS on intracellular compartments and organelles. Radiation-induced genomic instability is more likely to be associated with epigenetic mechanisms and small adjustments made in the DNA bases, therefore affecting the whole genome (Morgan et al., 1996).
Genomic instability has been characterized as a hallmark of cancer due to the multiple alteration occurring in the genome. High-level microsatellite instability (MSI) and chromosomal instability (CIN) are two identified molecular principles observed in tissues from colon cancer. In the malfunction of DNA mismatch repair genes; often the silencing of MLH1 gene by promoter methylation, MSI exhibits mutations micro-satellites; a short-repeated DNA sequences. Whereas CIN displays aneuploidy where there is a loss in tumour suppressor genes such as TP53 and APC responsible in regulated cells growth and death. Nonetheless, both mechanism share a mutation in the BRAF gene (accountable for intrinsic signals involving direct cell growth) or CpG island methylator phenotype (CIMP) which is important in gene inactivation in cancer cells (Kaiser, Meckbach and Jacob, 2014).
Furthermore, RIBE and RIGI are thought to be interlinked as they elevate the up-regulation of oxidative stress in bystander cells similarly (Kadhim and Hill, 2015). Nonetheless, it is to be noted that RIGI responses is higher at low radiation dosages compared to higher dosages (Kadhim et al., 2013) therefore, suggesting that low-dose long-term fractionated radiation induces chronic oxidative stress. This oxidative stress is stimulated by the increase of mitochondrial ROS as seen with in RIBE, due to the insufficiency of an antioxidant that protects cells against oxygen toxicity mediated by ROS known as GSH which loses its function after repeated low-doses of ionizing radiation (Shimura et al., 2016). Mitochondrial damage is thought to be more extensive and persistent as it lacks histone protection and efficient DNA repair systems unlike nuclear DNA, in fact, mDNA contains polymerase γ (Pol γ) enzyme that repairs base substitution efficiently through exonucleolytic proofreading, however it has a low frameshift fidelity for repetitive sequences longer than 4 nucleotides (Longley et al., 2001). Ionizing radiation tends to impair the function of the mitochondria through the dysfunction of the electron transport chain thus increasing the production of reactive oxygen species. ROS damage to the molecules affects cell cycling, oxidizing PP2A on cysteine residues, and downregulating PP2A activity in long-term FR cells. The loss of PP2A activity leads to the loss of negative feedback control of the AKT pathway resulting in persistent AKT activity in cells after long-term FR. This consistent activation causes the stabilization of nuclear cyclin D1 by inhibiting its deregulation by GSK3β. The abnormal accumulation of cyclin D1 during the S phase perturbs DNA replication and suppression of replication fork progression thus leading to DSBs. This will therefore accelerate senescence and act as a developer to tumorigenesis (Shimura and Kunugita, 2016).
The adaptive response of ionizing radiation
A phenomenon has been raised in which cells have established a resistance mechanism when induced with radiation exposure at low doses in order to better handle the subsequent exposure to high doses (Kadhim, Moore and Goodwin, 2004). This phenomenon has attributed to the hypothesis that resistance mechanism at low doses will induce adequate chromosome break repair mechanism present at the time of high dose exposure leading to less residual damage (Wolff, 1998). However, these effects vary depending on the low linear energy transfer or radiation, dose and dose rate. It has been discovered that adaptive response is dependent on proteins synthesized in the DNA damage response, stress response and the upregulation of cytokine signalling pathways (Kadhim, Moore and Goodwin, 2004). At low dose radiation exposure, the upregulation of protein kinase C through p38 MAP kinase stimulates the activation of P53 (Zhao et al., 2015); a tumour suppressor protein that has an essential role in the adaptive response, specifically in regulating radiation induced DSBs (Sasaki et al., 2002). whereas at higher doses, ERK and JNK kinases and WIP phosphatase are activated and induce the p38 MAP kinase pathway in order to increase the production of P53 protein, thus playing a role in the adaptive response (Lanza et al., 2005; Zhao et al., 2015).
The aim of the project
This study will involve experiments with four types of cancer cell lines, MDA, MCF7, CaCo2 and FSF. Where they will be cultured and irradiated with a 2Gy X-ray radiation at a dose rate of 0.525 Gy per minute; at the University of Oxford hospital, Gray institute for Radiology, Biology and Oncology. These irradiated cells are then to be tested for cell viability using a cell viability machine and chromosomal analysis under the microscope in order to observe the damage caused by the ionizing radiation. The media from the irradiated cells are to be transferred into normal fibroblasts cells to be checked for cell viability and chromosomal check for radiation induced bystander effect and genomic instability.
| Bystander mediator | Inhibitor | Effect upon BE induction | Reference |
| ROS | N-acetylcysteine (NAC) | Prevention of growth arrest | (Macip et al. 2002) |
| Cytokines i.e. TNF-α | Anti-sense oligonucleotides | Reduction in radiation-induced apoptosis | (M. Zhang et al. 2008) |
| Mitochondria | DNA depletion | Reduced γ-H2AX induction | (Chen et al. 2008) |
| Gap-junctions | Lindane/Octanol | Reduced p53 modulation/reduced mutagenesis | (Zhou et al. 2001; Azzam et al. 1998) |
| COX-2 | NS-398 | Reduced DNA damage | (Zhou et al. 2005) |
| Calcium | Calcicludine | Prevention of micronuclei induction | (Shao et al. 2006b) |
| Extracellular vesicles/ Exosomes | RNase A & heat (protein) | Abrogation of DNA damage mediation via an RNA/ Protein dependent mechanism | (Al-Mayah et al. 2012,2015; Jella et al, 2014) |
Reference list
Burtt, J. J., Thompson, P. A. and Lafrenie, R. M. (2016) ‘Non-targeted effects and radiation-induced carcinogenesis: a review’, J Radiol Prot, 36(1), pp. R23-35. doi: 10.1088/0952-4746/36/1/r23.
Chen, S., Zhao, Y., Zhao, G., Han, W., Bao, L., Yu, K. N. and Wu, L. (2009) ‘Up-regulation of ROS by mitochondria-dependent bystander signaling contributes to genotoxicity of bystander effects’, Mutat Res, 666(1-2), pp. 68-73. doi: 10.1016/j.mrfmmm.2009.04.006.
Desouky, O., Ding, N. and Zhou, G. (2015) ‘Targeted and non-targeted effects of ionizing radiation’, Journal of Radiation Research and Applied Sciences, 8(2), pp. 247-254. doi: https://doi.org/10.1016/j.jrras.2015.03.003.
Goodhead, D. T. (1994) ‘Initial events in the cellular effects of ionizing radiations: clustered damage in DNA’, Int J Radiat Biol, 65(1), pp. 7-17.
Hall, E. J. (2009) ‘Is there a place for quantitative risk assessment?’, J Radiol Prot, 29(2a), pp. A171-84. doi: 10.1088/0952-4746/29/2a/s12.
Hu, B., Grabham, P., Nie, J., Balajee, A. S., Zhou, H., Hei, T. K. and Geard, C. R. (2012) ‘Intrachromosomal changes and genomic instability in site-specific microbeam-irradiated and bystander human-hamster hybrid cells’, Radiat Res, 177(1), pp. 25-34.
Jackson, S. P. and Bartek, J. (2009) ‘The DNA-damage response in human biology and disease’, Nature, 461(7267), pp. 1071-8. doi: 10.1038/nature08467.
Jiang, Y., Chen, X., Tian, W., Yin, X., Wang, J. and Yang, H. (2014) ‘The role of TGF-β1–miR-21–ROS pathway in bystander responses induced by irradiated non-small-cell lung cancer cells’, Br J Cancer, 111(4), pp. 772-80. doi: 10.1038/bjc.2014.368.
Kadhim, M., Salomaa, S., Wright, E., Hildebrandt, G., Belyakov, O. V., Prise, K. M. and Little, M. P. (2013) ‘Non-targeted effects of ionising radiation–implications for low dose risk’, Mutat Res, 752(2), pp. 84-98. doi: 10.1016/j.mrrev.2012.12.001.
Kadhim, M. A. and Hill, M. A. (2015) ‘Non-targeted effects of radiation exposure: recent advances and implications’, Radiat Prot Dosimetry, 166(1-4), pp. 118-24. doi: 10.1093/rpd/ncv167.
Kadhim, M. A., Moore, S. R. and Goodwin, E. H. (2004) ‘Interrelationships amongst radiation-induced genomic instability, bystander effects, and the adaptive response’, Mutat Res, 568(1), pp. 21-32. doi: 10.1016/j.mrfmmm.2004.06.043.
Kaiser, J. C., Meckbach, R. and Jacob, P. (2014) ‘Genomic instability and radiation risk in molecular pathways to colon cancer’, PLoS One, 9(10), pp. e111024. doi: 10.1371/journal.pone.0111024.
Lanza, V., Pretazzoli, V., Olivieri, G., Pascarella, G., Panconesi, A. and Negri, R. (2005) ‘Transcriptional response of human umbilical vein endothelial cells to low doses of ionizing radiation’, J Radiat Res, 46(2), pp. 265-76.
Longley, M. J., Nguyen, D., Kunkel, T. A. and Copeland, W. C. (2001) ‘The fidelity of human DNA polymerase gamma with and without exonucleolytic proofreading and the p55 accessory subunit’, J Biol Chem, 276(42), pp. 38555-62. doi: 10.1074/jbc.M105230200.
Meek, D. W. (2009) ‘Tumour suppression by p53: a role for the DNA damage response?’, Nat Rev Cancer, 9(10), pp. 714-23. doi: 10.1038/nrc2716.
Morgan, W. F., Day, J. P., Kaplan, M. I., McGhee, E. M. and Limoli, C. L. (1996) ‘Genomic instability induced by ionizing radiation’, Radiat Res, 146(3), pp. 247-58.
Morgan, W. F. and Sowa, M. B. (2015) ‘Non-targeted effects induced by ionizing radiation: mechanisms and potential impact on radiation induced health effects’, Cancer Lett, 356(1), pp. 17-21. doi: 10.1016/j.canlet.2013.09.009.
Najafi, M., Fardid, R., Hadadi, G. and Fardid, M. (2014) ‘The Mechanisms of Radiation-Induced Bystander Effect’, J Biomed Phys Eng, 4(4), pp. 163-72.
Orth, M., Lauber, K., Niyazi, M., Friedl, A. A., Li, M., Maihöfer, C., Schüttrumpf, L., Ernst, A., Niemöller, O. M. and Belka, C. (2014) ‘Current concepts in clinical radiation oncology’, Radiat Environ Biophys, 53(1), pp. 1-29. doi: 10.1007/s00411-013-0497-2.
Pazhanisamy, S. K., Li, H., Wang, Y., Batinic-Haberle, I. and Zhou, D. (2011) ‘NADPH oxidase inhibition attenuates total body irradiation-induced haematopoietic genomic instability’, Mutagenesis, 26(3), pp. 431-5. doi: 10.1093/mutage/ger001.
Sasaki, M. S., Ejima, Y., Tachibana, A., Yamada, T., Ishizaki, K., Shimizu, T. and Nomura, T. (2002) ‘DNA damage response pathway in radioadaptive response’, Mutat Res, 504(1-2), pp. 101-18.
Shimura, T. and Kunugita, N. (2016) ‘Mitochondrial reactive oxygen species-mediated genomic instability in low-dose irradiated human cells through nuclear retention of cyclin D1’, Cell Cycle, 15(11), pp. 1410-4. doi: 10.1080/15384101.2016.1170271.
Shimura, T., Sasatani, M., Kamiya, K., Kawai, H., Inaba, Y. and Kunugita, N. (2016) ‘Mitochondrial reactive oxygen species perturb AKT/cyclin D1 cell cycle signaling via oxidative inactivation of PP2A in lowdose irradiated human fibroblasts’, Oncotarget, 7(3), pp. 3559-70. doi: 10.18632/oncotarget.6518.
Smith, J., Tho, L. M., Xu, N. and Gillespie, D. A. (2010) ‘The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer’, Adv Cancer Res, 108, pp. 73-112. doi: 10.1016/b978-0-12-380888-2.00003-0.
Streffer, C. (2000) Genomic instability induced by ionising radiation. Available at: http://www.irpa.net/irpa10/cdrom/01314.pdf.
Timmerman, R., Paulus, R., Galvin, J., Michalski, J., Straube, W., Bradley, J., Fakiris, A., Bezjak, A., Videtic, G., Johnstone, D., Fowler, J., Gore, E. and Choy, H. (2010) ‘Stereotactic body radiation therapy for inoperable early stage lung cancer’, Jama, 303(11), pp. 1070-6. doi: 10.1001/jama.2010.261.
USNRC Technical Training Center (2017) Natural and man-made radiation sources. Available at: https://www.nrc.gov/reading-rm/basic-ref/students/for-educators/06.pdf.
Wolff, S. (1998) ‘The adaptive response in radiobiology: evolving insights and implications’, Environ Health Perspect, 106(Suppl 1), pp. 277-83.
Xu, S., Wang, J., Ding, N., Hu, W., Zhang, X., Wang, B., Hua, J., Wei, W. and Zhu, Q. (2015) ‘Exosome-mediated microRNA transfer plays a role in radiation-induced bystander effect’, RNA Biol, 12(12), pp. 1355-63. doi: 10.1080/15476286.2015.1100795.
Yakovlev, V. A. (2015) ‘Role of nitric oxide in the radiation-induced bystander effect’, Redox Biol, 6, pp. 396-400. doi: 10.1016/j.redox.2015.08.018.
Yamamori, T., Inanami, O., Nagahata, H., Cui, Y. and Kuwabara, M. (2000) ‘Roles of p38 MAPK, PKC and PI3-K in the signaling pathways of NADPH oxidase activation and phagocytosis in bovine polymorphonuclear leukocytes’, FEBS Lett, 467(2-3), pp. 253-8.
Yamamori, T., Yasui, H., Yamazumi, M., Wada, Y., Nakamura, Y., Nakamura, H. and Inanami, O. (2012) ‘Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint’, Free Radic Biol Med, 53(2), pp. 260-70. doi: 10.1016/j.freeradbiomed.2012.04.033.
Zhao, Y., Zhong, R., Sun, L., Jia, J., Ma, S. and Liu, X. (2015) ‘Ionizing Radiation-Induced Adaptive Response in Fibroblasts under Both Monolayer and 3-Dimensional Conditions’, PLoS One, 10(3). doi: 10.1371/journal.pone.0121289.
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more