Abstract
One of the most drastic adaptations our species must survive is the physiologic transition from intrauterine life to extrauterine life. Prematurity presents extraordinary challenges in oxygenation and hemodynamic stability for those infants born too early. The immaturity of the premature heart, lungs, and brain require advanced technology to support healthy growth and normal development in the neonatal intensive care unit and beyond. Early, accurate, individualized, and timely recognition and treatment of circulatory or pulmonary deterioration is essential to support systemic blood flow to the brain and vital organs. Neonatal nurses require continuous, real-time, and accurate data on hemodynamics to ascertain critical clinical information to guide subsequent nursing care, assess responses to interventions, and understand the impact of the intensive care on the unique cardiopulmonary system in various stages of development. Electrical cardiometry (EC) is a technology that provides a more detailed, continuous, and timely measurement of hemodynamic status than current monitoring strategies of heart rate, blood pressure, and saturated pulse oximetry. Optimal respiratory support must balance the delicate developing pulmonary architecture, risks of oxygen delivery, and oxygenation with hemodynamics. The aim of this study is to examine electrical cardiometry measurements assessing the hemodynamic effects of mechanical ventilation in premature infants in a Level III neonatal intensive care unit.
Keywords: bioimpedance, cardiac output, electrical cardiometry, electrical velocimetry, hemodynamics, mechanical ventilation, premature infant, stroke volume, thoracic electrical bioimpedance
Chapter 1
The Problem
Despite advances in survival and knowledge accurate prediction of short- and long-term outcomes of the premature infant survivor are elusive and often remain hidden upon discharge from the neonatal intensive care unit (Johnston, et al., 2014). Recent advances such as therapeutic hypothermia, noninvasive ventilation, early surfactant administration, and umbilical cord milking have dramatically improved care and survival. However, these remarkable improvements are unable to predict or reduce chronic lung disease, intraventricular hemorrhage, and the major and minor co-morbidities of the premature infant (Islam, et al., 2015; Ment, et al., 2015). Technology continues to grow and expand, integrating more parameters for improved outcomes and is now reaching the smallest of premature infants under intensive care.
The growing focus on patient-driven evidence-based practice, outcomes, and long-term sequela require neonatal nurses to base clinical decisions on the most accurate and best available evidence. The bedside neonatal nurse provides continuous assessment and care and is often the first to note a change or deterioration in the status of the premature infants. Nursing assessment of physiologic data and behavioral response to treatment requires accurate, timely, and readily available measurements to adjust therapy or to relay to other members of the healthcare team for further investigation or evaluation. Current neonatal nursing assessment is sufficient during routine care of the stable premature infant, but has grave limitations when applied to the same infant in cardiopulmonary failure. Cardiopulmonary failure requiring mechanical ventilation magnifies the intensity of neonatal nursing care and requires critical care nursing skills and clinically relevant assessment integrating hemodynamic evaluation. EC is a technology validated against echocardiography, which is the current standard of care for hemodynamic assessment in premature infants, but its limitations restrict its applicability to the neonatal nurse (Kleinman & Seri, 2012). The aim of this study is to examine electrical cardiometry measurements, assessing the hemodynamic effects of mechanical ventilation, in premature infants in a Level III neonatal intensive care unit.
To frame this study, Chapter 1 describes the morbidity and mortality of prematurity including the burden of respiratory distress and cardiopulmonary compromise. The history of electrical cardiometry provides the infrastructure to synthesize the theoretical framework, conceptual model, relationships with the constructs, and definitions of terms. The theoretical underpinnings support the approach to the problem and research hypothesis. The chapter includes the assumptions and limitations of the proposed study and concludes with the significance to nursing practice and potential contribution to the care of the premature infant.
Incidence and Prevalence
Premature birth is a major health problem throughout the world and encompasses all infants born before 37 weeks gestation (Centers for Disease Control, 2016; World Health Organization, 2016). In 2015, premature birth surpassed all other causes of death in children under five and became the leading cause death in children worldwide (World Health Organization, 2016). Every year, in the world, fifteen million infants or one in every 10 is born premature (Neonatal Resuscitation Program, 2015; World Health Organization, 2016). In the United States, an estimated 9.6 % of all live births for 2015 were premature (March of Dimes, 2016). Prematurity is a significant risk factor for morbidity and mortality among infants contributing to considerable societal burden and economic costs (Blencowe, et al., Cousens, 2013; Johnston, et al., 2014; World Health Organization, 2016).
The Burden of Respiratory Distress
Respiratory distress syndrome is the most frequent admitting diagnosis and the primary cause of mortality in premature infants affecting 15-44% of neonatal intensive care admissions in the United States (Blencowe et al., 2012; Islam et al, 2015). Elective noninvasive respiratory support is a common strategy, but has a high failure rate among premature infants such that invasive, mechanical ventilation using positive pressure ventilation is required (Katheria & Leone, 2013; Shangle, et al., 2012; Vitali, et al, 2014). Premature infants who receive mechanical ventilation are at increased risk of oxidative and mechanical injury, inflammation, and further cardiovascular instability (Aversa, et al, 2016; Islam, et al, 2015; Kellar, Aschner, Hartert, & Moore, 2015).
The Burden of Cardiopulmonary Compromise
Cardiopulmonary compromise is a common finding in a large percentage of premature infants with a high incidence of mortality and significant associated short- and long-term morbidities (Blencowe, et al., 2012; Maitre, et al., 2015; Su, et al., 2016). Conceptually hemodynamics is an essential component of cardiovascular physiology dealing with the forces required to circulate blood through the cardiovascular system to provide an adequate supply of oxygen to the tissues and organs (Medical Dictionary, 2013). These hemodynamic forces include changes in filling pressures or preload, ventricular emptying or afterload, fluids in the thoracic cavity or thoracic fluid content, and contractility of the heart measured as increased or decreased heart rate (Ciccone, et al., 2011; Jobe, 2016). The goal of hemodynamic monitoring is to support tissue oxygenation and perfusion (Kleinman & Seri, 2012). Hemodynamic measurements are critical to the assessment and guidance of therapeutic decisions providing vital insight into systemic oxygen delivery and organ perfusion. Neonatal nurses require accurate measurements of hemodynamics to assess and monitor approaches to care and the individualized responses of premature infants in a variety of developmental stages.
Electrical Cardiometry
EC evaluates the electrical impedance of the heart permitting beat-to-beat evaluation of the premature infant’s overall hemodynamic status. The theoretical underpinnings of EC build on the work of Nyboer (1950) and Kubicek et al. (1966). EC estimates blood flow in the aorta by measuring pulsatile changes in thoracic impedance through four surface electrodes placed on the premature infant’s thorax. EC integrates Ohm’s law, which measures the opposition of body tissue to the flow of low magnitude, high frequency alternating current (Lukaski, 2013). EC provides a more accessible and direct measure of hemodynamic status than current clinical observation, assessment, and standard vital signs. EC measurements include measures of blood flow (heart rate, stroke volume and cardiac output), resistance (systemic vascular resistance), contractility (index of contractility, systolic time ratio, pre-ejection period, left-ventricular ejection time) and fluid (stroke volume variation, thoracic fluid content, correct flow time).
Problem
The immaturity of the myocardium contributes to changes in contractility, vascular tone, tissue perfusion, and hemodynamics (Kleinman & Seri, 2012; Soleymani, Borzage, & Seri, 2012). In the premature infant, these considerable differences in structure and function can lead to further compromise by changes in mean airway pressure and end expiratory pressure from mechanical ventilation (Hausdorf & Hellwege, 1987; Kluckow & Evans, 1996; Polin, 2012; Lakkundi, Wright, & de Waal, 2014). Additionally, susceptibility to respiratory distress from premature lungs confounds the immaturity of the cardiovascular system with decreased oxygen supply leading to poor cardiac output. Collectively, this results in alterations in the blood flow, and pumping ability and contractile forces of the heart measured by changes in impedance through EC. Several authors indicate that alterations in cardiac output may be one of the contributing factors associated with alterations in cerebral blood flow and the increased incidence of intraventricular hemorrhage, impaired neurodevelopmental outcomes, and mortality (Brew, Walker, & Wong, 2014; Gilmore, et al., 2011; Madzwamuse, et al., 2015; Noori, et al., 2014; Polin, 2012). There is an inverse relationship between the risk of morbidity and mortality and gestational age. The earlier the premature infants is born, meaning the lower the gestational age, the higher the risk of hemodynamic compromise and death, necessitating the control of gestational age as a confounder.
Current Standard of Care
Echocardiography is the current gold standard for assessing hemodynamics in the premature infant (Kleinman & Seri, 2012). Limitations of echocardiography include the fact that it is an isolated measurement of cardiac function, requires skilled interpretation, carries associated costs, must be interpreted by a cardiologist, and is technically challenging (Grollmuss, et al., 2012; Grollmus & Gonzalez, 2014; Song, et al., 2014; Torigoe, Sato, Nagayama, & Yamazaki, 2015). In contrast, EC provides a continuous measurement of multiple aspects of hemodynamics that provide the bedside neonatal nurse with real-time measures of hemodynamic performance. Several advantages of EC reported in the literature are the ease of use, availability, feasibility, and agreement with echocardiography (Grollmuss, et al., 2012; Song, al., 2014; Torigoe, et al., 2015). EC has demonstrated good correlation with thermodilution techniques, transthoracic and transesophageal echocardiography in premature infants (Tomaske, et al., 2009; Zormena, et al., 2007). Thermodynamic techniques are invasive and contraindicated in premature infants prone to infection and in whom small size prohibits their use. EC has shown reliability during hemodynamic alterations during cardiovascular surgery in premature infants (Lien, Hsu, Chu, & Chang, 2014). Current nursing standards of care for hemodynamic monitoring including bedside physical assessment, heart rate, blood pressure, and oxygen saturation. These measurements do not provide the neonatal nurse with timely, accurate, and objective data directly related to hemodynamic status (Bohn, 2011; de Boode, 2010). Current neonatal nursing practice does not include echocardiography or EC.
Hemodynamic Assessment
Current nursing care for the medically fragile or critically ill premature infant includes the assessment and interpretation of indirect measures of hemodynamic response including noninvasive blood pressure, heart rate, urine output, saturated pulse oximetry, and capillary refill time. Several authors have shown that these clinical assessment findings of hemodynamic status are inaccurate (Kluckow & Evans, 1996; Luce, Hoffman, & Bauer, 2007; de Boode, 2010). Hemodynamic response and abnormalities are more variable in the neonatal population secondary to differences in cardiovascular anatomy, maturational differences related to organogenesis, and varying response to treatment (de Boode, 2010). A gap in the literature is whether objective continuous measures of hemodynamic status could guide neonatal nursing care, assessment, and management in an effort to reduce morbidity and mortality secondary to cardiovascular dysfunction and eventual circulatory failure.
Mechanical Ventilation and Hemodynamics in Premature Infants
There are a limited number of published research studies investigating the hemodynamic effects, assessed through EC, of mechanical ventilation on premature infants. EC studies of the hemodynamic response during mechanical ventilation in the premature infant are lacking. Complicating this finding are the variable responses of various gestational ages and the observation that physical assessment findings are poor indicators of systemic blood flow. Available studies evaluating hemodynamics during mechanical ventilation in premature infants are lacking; current modes of ventilation highlighting the current gap in knowledge even more. Data are lacking related to how mechanical ventilation affects key factors associated with hemodynamic performance in the premature infant. The lack of recent literature includes the hemodynamics effects, assessed through any means, in mechanically ventilated premature infants and alterations in the contractility, blood flow through the pulmonary vasculature, hearts filling pressures, and fluids in the lungs (Ciccone, et al., 2011; Jobe, 2016).
Limitations of EC studies in the literature include small sample sizes and limited comparison in unstable premature infants, with much of the literature reporting observational findings of stable premature infants (Kamath, et al., 2011). The mechanisms of many of the catastrophic outcomes related to mechanical ventilation remain unknown (Albertine, 2012). It is unknown whether positive pressure ventilation has relevant hemodynamic changes that would affect nursing care, assessment, or interventions (Song, et al., 2014). Objective monitoring would provide the bedside nurse with vital information on which to base therapeutic strategies to minimize the current devastating and costly short- and long-term outcomes, communicate relevant data to other members of the healthcare team driving further investigation, and changes in the current plan of care.
Despite all we know, contemporary perinatal and neonatal researchers are urging research to develop approaches to care to reduce the burden of short- and long-term morbidities and mortality (Cayabyab, McLean, & Seri, 2009; Grollmus & Gonzales, 2014; Kleinman & Seri, 2012). Further validation of EC in premature infants is needed (Kleinman & Seri, 2012; Soleymani, Borzage, & Seri, 2012). Although significant advances in the pulmonary management of the premature infant are currently being implemented, further investigation is needed to evaluate the efficacy, impact, and other relevant outcome measures (Bancalari, & Calure, 2015; Cayabyab, McLean, & Seri, 2009; Grollmus & Gonzales, 2014; Kleinman & Seri, 2012). Translational research focusing on hemodynamic measurements that include developmental cardiopulmonary assessment, interventions, and outcomes is critical (Azhibekov, Noori, Soleymani, & Seri, 2013). Improved measures of hemodynamic function have the potential to guide assessment, management, and potentially reduce morbidity and mortality secondary to potentially harmful interventions and unrecognized decompensation (Azhibekov, et al., 2013; Boet, Joudain, Demontoux, & De Luca, 2016). Early, accurate, and timely recognition and treatment of circulatory or pulmonary deterioration is essential to support systemic blood flow to the brain and vital organs (Bohn, 2011; Kleinman & Seri, 2012). Hemodynamic measurements that provide objective criteria to assess systemic blood flow, organ perfusion, oxygenation, and cardiovascular compromise are essential for the appropriate treatment of premature infants (Carlisle, et al., 2010; Evans, 2016; Gillmore, et al., 2011; Noori, et al., 2012; Polin, 2012). In summary, no current study was identified investigating the effect of mechanical ventilation on hemodynamic performance in premature infants that might guide or augment neonatal nursing care. No studies were found using EC in neonatal nursing.
Purpose
The purpose of this prospective observational single center investigation is to characterize the hemodynamic effects, assessed through EC, of mechanical ventilation on premature infants in a Level III neonatal intensive care unit. Outcome measures include EC measurements of hemodynamic parameters potentially affected by mechanical ventilation including measures of contractility, flow, fluid, and resistance. The analysis will examine the relationship between hemodynamic measurements assessed through EC and mechanical ventilation in premature infants controlling for gestational age. The goals of this study are to contribute to the body of knowledge on EC and provide evidence for future clinical guidelines for practice. Improved knowledge of premature infant hemodynamics during mechanical ventilation may provide additional value to neonatal nursing’s understanding of the pathogenesis of hemodynamics during mechanical ventilation and improve neonatal nursing clinical assessment, intervention, and care.
Research Hypothesis
Limited research exists on the hemodynamic effects, assessed through EC, of mechanical ventilation on the premature infant controlling for gestational age. This study hypothesizes that using EC for hemodynamic measurement during mechanical ventilation will enable circulatory physiological conditions from mechanical ventilated to be interpreted and identified. This research study includes three aims with three null and alternative hypotheses.
Aim 1: To evaluate the relationship between the hemodynamic effects assessed through electrical cardiometry, of mechanical ventilation in premature infants, on the probability of death from respiratory failure.
H0: Electrical cardiometry will demonstrate no relationships between the hemodynamic measurements of mechanical ventilation and the probability of death from respiratory failure, controlling for gestational age
H1: Electrical cardiometry will demonstrate a relationship between the hemodynamic measurement of mechanical ventilation and probability of death from respiratory failure with an inverse relationship to gestational age.
Aim 2: To evaluate the correlation between gestational age and indicators of hemodynamic decompensation in mechanical ventilated premature infants using electrical cardiometry.
H0: Electrical cardiometry derived hemodynamic measurements of mechanically ventilated premature infants will not demonstrate a correlation between gestational age and indicators of hemodynamic decompensation in contractility, flow, fluid, and/or resistance.
H1: Electrical cardiometry derived hemodynamic measurements of mechanically ventilated premature infants will demonstrate a correlation between gestational age and indicators of hemodynamic decompensation in contractility, flow, fluid, and/or resistance of one standard deviation unit from the mean.
Aim 3: To investigate the ability of electrical cardiometry measurements of hemodynamic function in premature infants receiving mechanical ventilation to predict outcomes while controlling for gestational age.
H0: Electrical cardiometry assessed hemodynamic function of mechanically ventilated premature infants will demonstrate no predictive value for oxygen dependence at 36 weeks postmenstrual age.
H1: Electrical cardiometry assessed hemodynamic function of mechanically ventilated premature infants will demonstrate a predictive value for oxygen dependence at 36 weeks postmenstrual age.
Theoretical Approach
The Model of Electrical Velocimetry (EV) explores the theoretical argument that there is a relationship between changes in erythrocyte orientation and measurements of contractility, flow, resistance and fluid, captured through EC measurements. Mechanical ventilation creates changes in pulmonary blood flow and intrathoracic pressure influencing the heart and great vessels. These changes in pressure and blood flow alter the orientation of erythrocytes creating alterations in cardiovascular function and blood in the thoracic cavity. The observation and description of neonatal hemodynamics under different physiologic conditions is essential to guide clinical application and subsequently nursing practice. The specific theory used in this investigation is the Model of Electrical Velocimetry (Osypka & Bernstein, 1999; Osypka, 2009). The Model of Electrical Velocimetry provides a logically structured representation of the concepts, variables, and relationships involved in this investigation with the purpose of clearly identifying the hemodynamic effects assessed through EC mechanical ventilation on premature infants controlling for gestational age.
History
Historically the term bioelectrical impedance applies to the electrical properties of living organisms and is a measure of the opposition to current or impedance through the tissues of the body (Khalil, Mohktar, & Ibrahim, 2014). The first use of electrical impedance measurements came from Dr. Nyboer in 1950 (Nyboer, 1950). Dr. Nyboer used impedance measurements to describe various physiologic parameters, including blood flow in the lower extremities (Nyboer, 1950). The initial research by Dr. Nyboer introduced the field of bioimpedance and plethysmography that measures changes in body fluids including blood (Nyboer, 1950). Dr. Kubicek and colleagues continued the research moving the science into the thorax and modifying the procedure to the four-electrode thoracic impedance model used today (Kubicek, et al., 1966). Dr. Kubicek and colleagues further developed the model of Dr. Nyboer using assumptions regarding the relationship of the impedance signal and changes seen in the waveform. Theoretically, Dr. Kubicek attributed signal changes to the expansion of the aorta and the Windkessel effect (Kubicek 1968). In this model, the velocity of blood flow moved in a radial direction displaying a signal he defined as a “volumetric change” in the aorta. “Blood is a strong electrical conductor and the signal studied fifty years ago is essentially the same signal used today” (M. Osypka, personal communication, October 6, 2016).
Model of Electrical Velocimetry
Current advances and improvement in processors and filters over the last decade, has allowed scientists to capture the small timing window of 30 to 90 milliseconds in which the aortic valve opens. These advances have contributed to a deeper understanding of the impedance signal and the current Model of Electrical Velocimetry (M. Osypka, personal communication, October 6, 2016). In 1999, Dr. Osypka and Dr. Bernstein reevaluated the assumptions and added new knowledge based on the alignment of erythrocytes. In the new Model of Electrical Velocimetry, volumetric changes in the ascending aorta produce the changes noted in the bioimpedance signal immediately after aortic valve opening (Osypka, 2009). By questioning the assumptions of the origin of the thoracic impedance signal, the current theoretical model was derived. The Model of Electrical Velocimetry utilizes the conductivity of the blood in the aorta and changes in aortic acceleration and blood flow in an axial direction versus radial direction. The newer model incorporates peak aortic acceleration instead of velocity to describe blood flow, timing, and signal origin. The current Model of Electrical Velocimetry captures the conductivity of erythrocytes, or red blood cells in the aorta during different phases of the cardiac cycle. Impedance is a measure of conductivity assessed by the electrical current of erythrocytes using EC (Osypka & Bernstein, 1999). EC targets aortic blood conductivity changes corresponding to the orientation of the erythrocytes from random orientation to one of alignment (Figure 1). EC extracts the conductivity changes due to alterations in blood conductivity to determine hemodynamic measurements. Limitations of the Model of Electrical Velocimetry include a lack of empirical validation through internal and external testing.
Figure 1: Theoretical Model of Electrical Velicimetry (Bernstien & Ospka, 1999).

Figure 1. Theoretical Model of Electrical Velicometry (Bernstien & Ospka, 1999) displaying a diagram of aortic blood conductivity changes corresponding to the extent of common orientation of the erythrocytes during the various flow states within the cardiac cycle. Erythrocytes during diastole are in random orientation and become aligned in orientiation during systole. Electrical Cardiometry extracts the conductivity change due to changes in blood conductivity that are used to produce hemodynamic waveforms and measurements.
Concepts and Constructs
Hemodynamic monitoring concentrates on the cardiopulmonary system and end organ perfusion. Markers such as blood pressure and heart rate provide indirect measures of the cardiovascular function (de Boode, 2010). Surrogates of organ perfusion including capillary refill, urine output, lactate level, and mental state are not accurate, delayed, or require blood sampling (de Boode, 2010). EC permits advanced hemodynamic monitoring of the components that affect the cardiovascular system including preload, afterload, fluid status, and contractility. In the premature infant, EC is the most logical choice to capture cardiopulmonary compromise, and offers a more accurate assessment of hemodynamic function than standard physical assessment and vital signs.
Relationships between Constructs
The conceptual model (Figure 2) displays the cardiopulmonary changes associated with mechanical ventilation that contribute to the effects, integration of the theoretical framework, hemodynamic monitoring using EC, and the existing or potential directional relationships between the hemodynamic key concepts, and associated measurements. Outcome measures include EC measurement of hemodynamic parameters potentially affected by mechanical ventilation including the key concepts of afterload, contractility, preload, and thoracic fluid content. Alterations in cardiovascular function manifest as changes in filling pressures or preload, ventricular emptying or afterload, fluids in the thoracic cavity or thoracic fluid content, and contractility of the heart measured as increased or decreased heart rate (Ciccone, et al., 2011; Jobe, 2016). The effects of these variations in the distribution of thoracic blood volume as a factor of heart function alter thoracic impedance. The measures captured and derived through EC link the core concepts to changes in contractility, flow, resistance, and fluid. In preterm infants, cardiac output is higher than adults and is the product of heart rate and stroke volume which depends on myocardial contractility, preload, and afterload which affect thoracic fluid (Frank-Starling mechanism) (Kleinman & Seri, 2012). Thoracic fluid content (TFC) is an assessment of fluid status and changes, especially in the lungs (Lee, et al., 2015). In premature infants, differences in myocardial tissue lower compliance and contractility and reduce the role of preload in determining stroke volume although there is limited published research to compare these indices differences outside of animal models. Contractility, flow, resistance, and pressure capture the measurements reflective of the key concepts. This investigation will explore the hemodynamic effects assessed through EC of mechanical ventilation on hemodynamic measurements while examining covariates that may predict, confound, or interact with the independent and dependent variables. The covariates in the conceptual model include APGAR scores, gestational age, gender, height, hemoglobin level, medications, noninvasive blood pressure, race, and weight.
Mechanical ventilation influences intrathoracic pressure and blood flow in the pulmonary vasculature (Jobe, 2016). Mechanical ventilation can be lifesaving or lead to injury depending on the interplay of the cardiopulmonary system and level of ventilatory support. In this descriptive study, key factors and relationships between them are isolated and identified in Figure 2. Increases in intrathoracic pressure alter the amount of blood in the thoracic cavity and increase resistance to pulmonary blood flow through lung distention and compression on the pulmonary vasculature and smaller capillaries (Alander, et al., 2013). The impact of mechanical ventilation additionally influences the heart and great vessels located within a closed chest cavity causing alterations in cardiovascular function (Jobe, 2016). Increases in lung volume translate to increased pressure against the heart and great vessels from the inflated lungs. Alterations in cardiovascular function manifest as changes in filling pressures or preload, ventricular emptying or afterload, and contractility of the heart measured as increased or decreased heart rate (Ciccone, et al., 2011; Jobe, 2016). The effects of these variations in the distribution of thoracic blood volume as a factor of heart function alter thoracic impedance. The Model of Electrical Velocimetry captures the changes in erythrocytes orientation, alignment, and conductivity using EC to examine measurements of cardiovascular function impacted by mechanical ventilation (Osypka, 2009).
Figure 2: Conceptual Model of Electrical Velocimetry (Osypka & Bernstein, 1999).
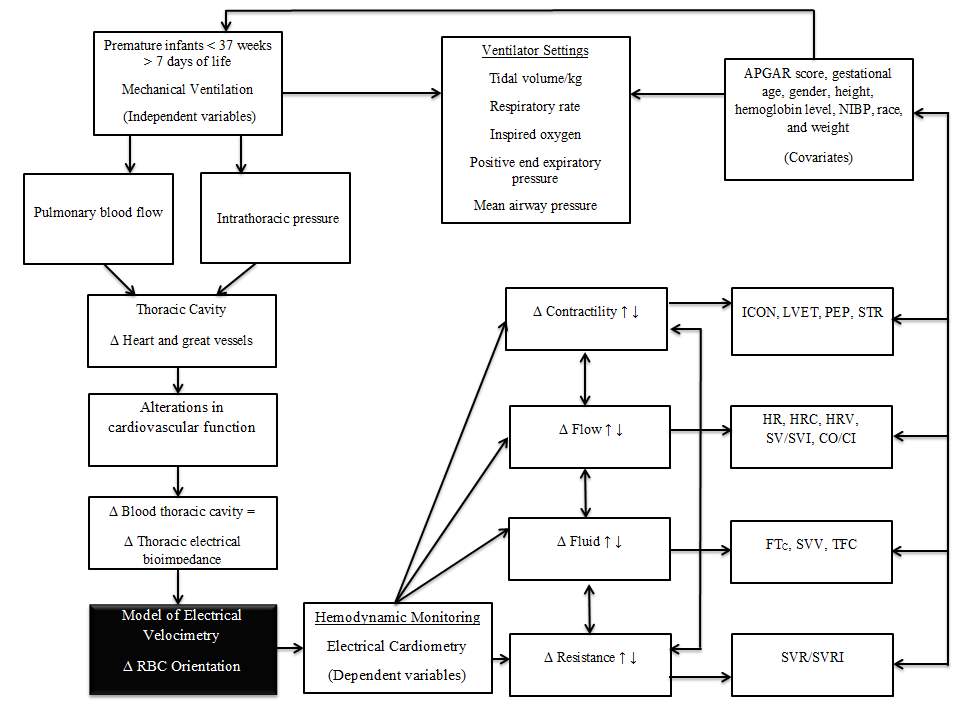
1 Δ = Change; ↑ = increase; ↓ = decrease; <=less than; > = greater than
Mechanical ventilation induces changes in intrathoracic pressure that are different from spontaneous ventilation. Premature infants in various stages of lung development are vulnerable to the reversed effects of ventilation (Alander, et al., 2013). Spontaneous ventilation decreases intrathoracic airway pressure creating a negative pleural pressure defined as a pressure below atmospheric pressure (Jobe, 2016). In contrast, mechanical ventilation uses positive pressure, which increases intrathoracic airway pressure above atmospheric (Jobe, 2016). Premature infants additionally require the use of positive end-expiratory pressure to prevent the collapse of the alveoli that further alters intrathoracic pressures. Ventilator settings in the conceptual model include medically prescribed properties programmed into the mechanical ventilator to support the premature infants underlying pathology and physiology. The specific ventilator settings in the conceptual model include those that may influence pulmonary blood flow, alter intrathoracic pressure, and potentially have an effect on the cardiovascular system. These ventilation settings include tidal volume, inspired oxygen percentage, positive end expiratory pressure, and mean airway pressure (Figure 2).
The conceptual model (Figure 2) framework drawn from current understanding of physiology and pathophysiology displays the influences of mechanical ventilation on hemodynamic measurements using EC. The Model of Velocimetry (Figure 1) proposes an explanation to detect any changes in thoracic impedance to test if a relationship exists (Bernstein & Osypka, 1999). Based on the Model of Velocimetry, study variables include hemodynamic measures that are congruent with the model and reflective of the intervention of mechanical ventilation. Mechanical ventilation leads to changes in the premature infant’s lung volume, pleural and thoracic pressure, and may have a direct effect on cardiovascular dynamics (Jobe, 2016). Dependent variables are EC hemodynamic measurements of cardiovascular functioning reflective of preload, afterload, fluid, and contractility. Theoretically, EC captures the alignment of erythrocytes during the different phases of the cardiac cycle. EC is sensitive to changes in flow, resistance, contractility, and fluid volume that connect the individual EC measurements to the model. This study will start the process of elucidating the potential consequences of mechanical ventilation on hemodynamics in the premature infant. The availability of objective measurements will enable researchers to examine neonatal hemodynamics in infants receiving mechanical ventilation.
Definitions
Defining all terms that are essential to the study is complex and interrelated. EC is a noninvasive means to measure thoracic electrical bioimpedance and provides a logical approach to understanding the cardiovascular component of assessment in the ventilated premature infant. The definitions in this study apply to all premature infants less than 37 weeks gestation. For clarity, the trademark symbol (®) is applied to the EC equipment called the Aesculon® or ICON® and mechanical ventilator. The index of contractility (ICON) measurement differs from the equipment used to obtain measurements by the trademark symbol.
Afterload
Conceptual definition. Afterload is the amount of resistance the left ventricle must pump against to eject the premature infant’s stroke volume (Osborn, Evans, & Kluckow, 2007). Afterload is inversely related to left ventricular contractility, systemic blood flow, perfusion to vital organs, and cardiac output in the premature infant (Osborn, et al., 2007).
Operational definition. Measurements of afterload are from theAesculon® or ICON®. OperationalizedEC capturesmeasurements of afterload or the physical forces the ventricles must overcome or generate to propel the stroke volume and cardiac output forward including resistance and the measurements of systemic vascular resistance (SVR) and SVR indexed (SVRI) to body surface area.
Cardiac Output/Cardiac Index
Conceptual definition. Cardiac output (CO) and cardiac index (CI), incorporating height and weight, is the amount of blood (in milliliters) ejected by the premature infant’s heart per minute and is dependent on heart rate, contractility, preload, and afterload which overlap as determinants of flow (Noori, Drabu, & Seri, 2012).
Operational definition. CO and CI are measured using the ICON® or Aesculon® as a continuous variable in milliliters (ml) per kilogram (kg) per minute (Azhibekov, et al., 2013).
Contractility
Conceptual definition. Contractility refers to the intrinsic force generated by the heart to “shorten myocardial fibers,” regardless of preload or afterload, to propel the blood forward (Bombardini, 2005). Contractility has a cascading effect on end-systolic volume in the heart and ultimately stroke volume and cardiac output, both indicators of hemodynamic performance.
Operational definition. Measurements of contractility are from theAesculon® or ICON®. Contractility includes hemodynamic parameters that reflect heart contractility measured through EC including the ICON, left ventricular ejection time (LVET), pre-ejection period (PEP), and systolic time ratio (STR). These parameters are reflective of systolic time intervals.
Corrected Flowtime
Conceptual definition. Corrected flowtime (FTC) is an indicator of preload or a ‘static’ preload indicator comparable to central venous pressure and left ventricular end-diastolic measurement pressure (Lee, et al., 2007).
Operational definition. FTC is measured as a continuous variable using the Aesculon® or ICON® relying solely on the timing of the cardiac cycle determined by LVET normalized for heart rate.
Electrical Cardiometry
Conceptual definition. EC is an impedance-based method for determining hemodynamic measurements in premature infants. EC determines these measurements by producing a waveform (impedance cardiogram) and extracting changes in the thoracic impedance caused by the heart rate and cardiac cycle.
Operational definition. EC measurement is through the Aesculon® or ICON®. Both pieces of equipment measure the same hemodynamic parameters. The ICON® is a handheld unit and the Aesculon® is a full bedside monitor.
Electrical Velocimetry
Conceptual definition. EV is a method of interpreting the bioimpedance signal developed by Bernstein and Osypka in 1999 using the impedance waveform.
Operational definition. The Aesculon® and ICON® monitors contain the EV methodology enabling use in premature infants.
Flow
Conceptual definition. Flow refers to blood propagation throughout the circulatory system derived through EC. EC provides the hemodynamic monitoring parameters reflective of blood flow potentially affected by mechanical ventilation.
Operational definition. Determinants of blood flow will be measured as a continuous variable using Aesculon® or ICON® and include measurements of CO and CI, heart rate (HR), heart rate complexity (HRC), heart rate variability (HRV), stroke volume (SV), and stroke volume index (SVI).
Fluid
Conceptual definition. Fluid refers to the electrical conductivity of the thorax body liquids captured through EC measuring the interstitial, intra-alveolar, and intravascular fluids.
Operational definition. Fluid is the derived hemodynamic measurements from the Aesculon® or ICON® measuring FTC, stroke volume variability (SVV), and thoracic fluid content (TFC).
Heart rate
Conceptual definition. HR is the number of contractions by the ventricles of the heart measured in beats per minute and is an indicator of contractility, stroke volume, and CO (Bernstein & Osypka, 1999).
Operational definition. HR is a direct continuous measure through theAesculon® or ICON® in beats per minute and is an indicator of blood flow.
Heart Rate Complexity
Conceptual definition. HRC measures cardiovascular R-to-R (from the QRS complex on the electrocardiogram) interval randomness or unpredictability.
Operational definition. HRC measurement is a continuous variable obtained from the Aesculon® or ICON®, and is a numeric without dimension (no specifying element such as percent or milliliter).
Heart Rate Variability
Conceptual definition. HRV measures cardiovascular R-to-R interval (RRI) oscillations and the heart rate beat-to-beat variability. HRV is a measure of the “autonomic nervous system’s influence on heart rate” and is a measure reflective of autonomic nervous system control (Brandle, et al., 2015).
Operational definition. HRV measurement is a continuous variable reflective of blood flow obtained through EC using the Aesculon® or ICON®, as a dimensionless numeric.
Hemodynamics
Conceptual definition. Hemodynamics is the study of the mechanics of blood flow as it relates to the cardiovascular and the circulatory systems. The pathophysiology of blood flow and heart function, directly relate to the physics of fluid dynamics or flow.
Operational definition. EC hemodynamic measurements from theAesculon® or ICON® provide quantifiable data representative of resistance, contractility, blood flow, and fluid status. Each of these interrelated outcome variables relates to an aspect of cardiovascular mechanics indicative of these concepts (Figure 2).
Index of Contractility
Conceptual definition. The ICON is a hemodynamic measurement derived by EC of contractility and an indicator of preload and intravascular volume. EC uses the peak amplitude divided by the base impedance as an index of aortic acceleration and an index of contractility (Osypka, 2009).
Operational definition. TheICON measurements from theAesculon® or ICON® is an indicator of contractility derived from the maximum rate of change of thoracic electrical bioimpedance reported as a dimensionless numeric.
Intrathoracic Pressure
Conceptual definition. Intrathoracic pressure is the force within the thoracic cavity that varies depending on the prescribed settings of the mechanical ventilator. Alterations in intrathoracic pressure can relate to the underlying pathophysiology, gestational age, and stage of lung development, or changes in ventilator settings that may affect cardiovascular function.
Operational definition. Intrathoracic pressure monitoring is through mean airway pressure in centimeters of water pressure (cm H2O pressure).
Left Ventricular Ejection Time
Conceptual definition. LVET is a hemodynamic measurement derived by EC that measures the time interval from aortic valve opening to closing and is a hemodynamic measure of left ventricular performance.
Operational definition. LVET is a derived measurement of theAesculon® or ICON® in milliseconds.
Mechanical Ventilation
Conceptual definition. Mechanical ventilation is any assisted breathing machine using positive pressure or volume to inflate the lungs that connects to the premature infant’s airway and for this study includes the use of the SERVO-n® neonatal ventilator by Maquet®.
Operational definition. These measurements are obtained from the mechanical ventilator and include tidal volume (TV) measured in ml per kg, respiratory rate (RR) measured as breaths per minute, inspired oxygen (FiO2) measured as a percent, positive end-expiratory pressure (PEEP) measured in centimeters, and mean airway pressure (MAP) measured in cm H20 pressure. These measurements indicate the prescribed settings guided by the premature infant’s pulmonary pathophysiology to assist or replace spontaneous breathing.
Preload
Conceptual definition. Preload is the end-diastolic volume directly related to ventricular filling pressure during cardiac diastole. Preload is interrelated with afterload and contractility to produce adequate CO (Gupta & Rosenkrantz, 2014). Factors that increase preload similarly increase stroke volume up to a point as determined by the Starling law (Gupta & Rosenkrantz, 2014). Preload is one of several determinants of stroke volume that affects cardiac output and eventually alters oxygen delivery to the tissues (Gupta & Rosenkrantz, 2014).
Operational definition. The Aesculon® or ICON® measures preload indices affecting fluid including the measurements of SVV, TFC, and FTC.
Premature Infant
Conceptual definition. Preterm birth is “the birth of an infant prior to 37 completed weeks of gestation” (World Health Organization, 2016). Prematurity for this study will include all infants less than 37 weeks gestation that are greater than seven days of age. The cut point of seven days of age is to reduce the impact of transitional cardiovascular shunts that could affect EC measurements.
Operational definition. The gestational age in weeks of the premature infant is from the electronic medical record and based on physical assessment of the premature infant and the mothers last menstrual cycle or postmenstrual age.
Resistance
Conceptual definition. Resistance is the amount of systemic dilation or constriction in the circulatory system and is a factor of afterload that in turn affects end-systolic volume and stroke volume.
Operational definition. Measurements of resistance from theAesculon® or ICON® include SVR and SVRI measured as a continuous variable in dynes per second.
Stroke Volume and Stroke Volume Index
Conceptual definition. SV and SVI are direct measurements of the amount of blood pumped by the left ventricle per heartbeat (Vergnaud, et al., 2015).
Operational definition. SV and SVI are continuous variables measured by theAesculon® or ICON® in milliliters per beat and milliliters per meter squared (Vergnaud, et al., 2015).
Stroke Volume Variation
Conceptual definition. SVV is a derived measure of blood flow. SVV is a hemodynamic measurement derived from the variation of stroke volume and an indicator of preload, optimal oxygen delivery, and fluid responsiveness.
Operational definition. SVV is a continuous variable measured by theAesculon® or ICON®,derived from the variation of stroke volume and measured as a percent (Soliman, Samir, Naggar, & Dehely, 2015).
Thoracic Fluid Content
Conceptual definition. Thoracic fluid content or TFC is an indicator of excess thoracic fluids or lung edema (Ciccone, et al., 2011). TFC is an EC derived hemodynamic measurement of the directional change of total fluid volume in the thorax derived through changes in thoracic conductivity. Changes in intrathoracic pressure and pulmonary blood flow from mechanical ventilation can affect TFC.
Operational definition. The Aesculon® or ICON® provides measurements of TFC. TFC is a continuous variable measured as thoracic fluid index (TFI) which is a dimensionless parameter.
Thoracic Electrical Bioimpedance
Conceptual definition. Thoracic electrical bioimpedance (TEB) is a noninvasive means of monitoring hemodynamic parameters. TEB measurements use four surface electrodes strategically placed on the left side of the body to create an external unperceivable electrical field applying an alternating electrical current to surround the ascending aorta and upper aspect of the descending aorta. The array of four electrodes permits the inner electrodes to measure the most significant changes in bioimpedance related to the orientation, alignment, and circulation of red blood cells based on the electrical signal waveform.
Operational definition. The TEB equipment to capture or derive EC hemodynamic measurements for this study includes the ICON® or AESCULON® by Osypka Medical and represents the only approved devises for use in premature infants (Osypka Medical, 2016).
Assumptions and Limitations
Assumptions
Several common assumptions relate to this investigation. These assumptions include:
3. Electrical Cardiometry provides continuous real-time data permitting assessment and reassessment of cardiovascular function across a continuum.
4. The study will take place in a Magnet facility and a Level III neonatal intensive care unit with an established research team. The Research using EC in this critical care area spans a decade.
5. Changes in care will not occur based on these electrical cardiometry measurements.
6. Electrical cardiometry monitoring is continuous.
7. The electrical cardiometry equipment runs through a self-check before connection to the patient. Biomedical Engineering verifies the equipment for accuracy and precision prior to the study and once a month. The EC monitors are on stands and independently run through a calibration before each study.
8. The mechanical ventilators run through a self-test before connection to the premature infant and monitored by a registered respiratory therapist.
Limitations
There are several limitations identified for this study. The primary limitations relate to controlling for confounding variables that could reduce internal validity. The population includes high-risk premature infants less than 37 weeks gestation with difficult medical courses. There are five limitations for this study.
1. Influencing factors include diagnosis (sepsis, intraventricular hemorrhages, and surgery), medications, family involvement, and differences in care providers. The study involves the application of inclusion and exclusion criteria, design, and statistical methodologies to minimize these factors.
2. The sample is a single center convenience sample in one large Southeastern Metropolitan area limiting generalizability.
3. Lastly, there is the matter related to reliable data collection and the application of the four impedance sensors between data collectors. Interrater reliability set above .90 demonstrates consistency among data collectors to control for this limitation. All data collectors have a minimum of one-year experience with electrical cardiometry.
Significance to Nursing
The aim of this study is to investigate the hemodynamic effects, assessed through EC, of mechanical ventilation in premature infants. Should the new measurements from this study demonstrate cardiovascular alterations unseen by current monitoring the next phase would involve replication in larger and different populations. Improved description of the effects of mechanical ventilation on cardiovascular function via EC offers neonatal nursing the capability to improve practice through the ability to anticipate, intervene, and potentially contribute to improved patient outcomes in this subgroup of the population.
Conclusions
The hemodynamic effects of mechanical ventilation on the premature infants of varying gestational ages is interrelated and complex. EC is a noninvasive means to measure thoracic electrical bioimpedance and provides a logical approach to understanding the cardiovascular component of assessment in the ventilated premature infant. Perhaps one of the most important unknowns is the effect of immediate and continuous noninvasive hemodynamic measurement of each unique infant under a variety of burdens and mechanical ventilation using data derived through EC on assessment, interventions, and current neonatal nursing practice.
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more