The Discovery and History of Graphene
The discovery of graphene has arguably been the biggest scientific breakthrough of this century. With its full capabilities not yet known, those who study and develop it continue to be amazed by its seemingly endless potential, although none more so than those who first discovered it. Graphene was brought to life in 2003 by physicist Andre Geim and his coworker Kostya Novoselov [1]. They were assisted by other contributors from both the Institute for Microelectronics Technology in Chernogolovka and the University of Manchester before eventually having their work recognized in a 2004 publication of Science. Six years later, in 2010, their scientific fame would reach its pinnacle when they received the Nobel Prize in Physics for the discovery and creation of graphene [2].
Their method was basic in practice and involved several iterations of using Scotch tape to peel away layers of graphite from an existing crystalline graphite structure until it was sufficiently thin enough to be characterized as a single layer thick. At the moment this was accomplished, graphene was born. It is important to note that while this was the first experiment to make graphene a measurable, analyzable reality, the concepts surrounding it were theoretically considered in relation to solid state physics in 1947, the wave equation in 1956, and the Dirac equation in 1984. Structures similar to graphene were also conceived in the 1960s, but there was no way to develop a single layer capable of being studied under any form of practical experimentation. The idea that this would ever be achievable was widely regarded as impossible. Following the work of Geim and Novoselov, graphene has burst onto the scientific scene and become the focus of a great deal of research. Dual layers of graphene have been investigated to determine how the addition of a single layer affects the characteristics of graphene, and studies on magnetic fields, mechanical properties, and light have all been applied to graphene [2].
Graphene And Its Properties
Following a brief history of graphene and its discovery, it is important to understand what graphene represents and the properties it possesses. At its core, graphene is, quite simply, a monolayer of graphite. This monolayer is comprised of carbon atoms arranged in a two-dimensional network structure that is hexagonal in shape [3, 4]. The boundaries of graphene’s lattice have been given their own names, armchair and zigzag (Fig. 1, [5]), due to their unique impact on electronic and magnetic qualities.

Fig. 1 – Armchair and Zigzag boundaries in monolayer graphene
In some situations, graphene can also be characterized as being bilayer or few-layer. This requires that the interpretation of graphene as a monolayer material alone be less strict, but this admission has led to several interesting revelations about how the number of layers impacts a variety of characteristics. Few-layer graphene is generally considered to have no more than ten layers, as beyond this point, electronic properties match those of graphite, basically making the material a graphite film [4]. Few-layer graphene has four different stacking arrangements, each of which have the potential to influence electronic properties. The least prevalent, but also the simplest, is the very ordered form of AAA stacking, in which each layer perfectly aligns with that above and below it. ABAB stacking, coined “Bernal Stacking”, and ABC stacking, “Rhombohedral”, are two other ordered forms (Fig. 2, [4]). 
Fig. 2 – Graphene Stacking Patterns
The final form is referred to as turbostratic and displays no discernible stacking order. It appears random and disorderly [4]. Aside from having multiple, three-dimensional stacked layer configurations, monolayer graphene can be manipulated into other shapes to represent each dimension in some form. It can be formed into zero-dimensional “buckyballs”, and rolled into one-dimensional nanotubes, as shown in Fig. 3 below.

Fig 3. – 0D, 1D, 2D, and 3D Congfigurations of Graphene [6]
Properties of Graphene
In terms of mechanical properties, graphene is, by all rights, the strongest material currently known to man. It has a Young’s Modulus measured at 1.0 TPa, and a stregnth of 130 GPa [6]. To put this massive amount of strength into perspective, it is about one-hundred times stronger than the stoutest form of steel [2]. Considering that graphene is at most only a few layers of atoms thick with an almost miniscule amount of mass, this becomes even more impressive from a strength-to-weight ratio outlook. As if this wasn’t astonishing enough, graphene’s thermal conductivity characteristics are virtually unparalleled as well. At values of around 5000 Wm-1K-1, even diamond’s thermal capabilities pale in comparison, let alone those of any other carbon nanomaterial or metal.
Graphene boasts many other intriguing qualities, namely a very large surface area of some transparency and the ability to maintain its electrical integrity even after a change in shape [5]. Graphene also possesses antibacterial properites. Although this area of study is relatively new and mostly unexplored for graphene, early studies have shown that both regular and reduced graphene oxide are capable of preventing the growth of bacteria. This revelation carries very beneficial implications considering that graphene and graphene oxide have been shown to have great biocompatibility, as stated by Chunhai Fan, a professor from Shanghai, in [6].
Despite its outstanding mechanical and thermal properties, graphene truly shines when it comes to electronics. Studies related to graphene’s electrical properties have been the greatest focus of research and revealed many fascinating truths about graphene’s one-of-a-kind nature. Graphene is, in essence, an outstanding electrical conductor, as evidenced by its ability to readily transport charge. Electrons move swiftly through graphene, exhibit almost massless behavior, and have been observed to not scatter as easily as in other materials. However, this last characteristic, better known as ballistic transport, has experimentally been shown to be restricted to small-scale samples of graphene. By contrast, bigger sheets of the material have diffusive qualities. This interesting phenomenon is likely due to flaws and imperfections in the larger graphene sheets which are amplified during macro-scale fabrication techniques [5]. Because of monolayer graphene’s single atom layer, electron waves moving through graphene are more easily observed with scanning probes. Finally, these waves also respond more readily to nearby circuitry components, making them great to use when studying circuits [6].
As mentioned earlier, the number, thickness, and orientation of graphene and its layers influences each of the properties listed above. Changes in thickness, in particular, can lead to either drastic or insubstantial changes in graphene’s properties. For example, electric-related qualities are only slightly susceptible to layer changes, whereas thermal conductivity is impacted significantly. Surface area, hardness, and physical aspects are impacted as well and all play a role in determining which form of graphene is most beneficial for an application [5].
Graphene Synthesis Methods
Now that the basics of graphene and its properties have been discussed, graphene synthesis methods need to be considered to develop an understanding of how such a revolutionary material can be made. It should be noted that while many new synthesis methods are being researched, there are some that have already been well established. As of now, small scale synthesis methods for graphene have developed widely and are feasible. Large scale methods, however, have proven to be inadequate from both a material properties and fiscal standpoint [6]. Much like other nanomaterials, graphene synthesis can be broken down into two major categories, top-down and bottom-up, which each have their own benefits and drawbacks. Top-down synthesis requires obtaining graphene layers by essentially peeling and breaking away multiple stacked layers. To do this, the van der Waals bonding present in the layers must be overcome. This task is easier said than done, and graphene sheets often leave the process damaged, resulting in low yields. Couple this with the fact that naturally occurring graphite is a limited substance, and it’s easy to see that top-down methods may not be the best bet if trying to preserve the world’s supply of graphite. By comparison, bottom-up synthesis creates graphene from carbon that exists outside of graphite. This is a relatively uncomplicated process but is plagued by the potential for poor material quality if not done properly. With this method, high temperatures need to be present to facilitate a successful synthesis that yields high-quality graphene [5].

Fig. 4 – Bottom-Up vs. Top-Down Synthesis [5]
Looking at top-down synthesis methods, perhaps the most straightforward is that which was used by Geim and Novoselov to first create it. Termed michromechanical cleavage, this method requires the repeated cleaving (exfoliation) of graphite layers, separating them until they eventually produce graphene. The amount of cleaving can be controlled to produce graphene with the desired number of layers. This method is particularly useful for producing a nice specimen for observation and study but is tedious to perform, limiting the overall scope of its use.
Other, more complicated methods of graphene synthesis involve exfoliation and are differentiated by the agent used to exfoliate the graphite. For example, in electrochemical exfoliation, graphite acts as the electrode in an electrolytic solution. The presence of various electrolytes serves to both exfoliate the graphene and to stabilize it. Because the electrolytes used can impact the graphene’s properties, care needs to be taken in selecting electrolytes that will yield the desired effect. Solvent exfoliation methods are also used and can involve the use of graphite intercalation compounds as well as sonication, a liquid agitation method spurred on by irradiation. Finally, to produce reduced graphene oxide, graphite oxide is exfoliated [5]. With the addition of heat, further exfoliation produces graphene sheets [6].
Non-exfoliation top-down methods include arc discharge and unzipping. With arc discharge, electric current is passed through graphite to produce graphene while in the presence of buffer gases, particularly those containing hydrogen. The hydrogen gas effectively eliminates extranneous bonding sites in the carbon, allowing the graphite sheets to retain their integrity and form. Unzipping, quite simply, utilizes chemical and physical methods to produce strips of graphene from carbon nanotubes [5].
Bottom-up methods are less numerous and more focused. One of the more prominent bottom-up methods is chemical vapor deposition. This practice uses metal substrates in conjunction with heat to break down gases that possess carbon. The break-down of the gas leads to the formation of graphene on the substrate. In other, alternative approaches to this, graphene can be synthesized from carbon-containing solids, rather than gases, or without the use of a substrate. Another major method is silicon carbide sublimation. With this synthesis technique, silicon carbide is heated at extremely high temperatures, subliming the silicon. The remaining carbon forms graphene, using either the Si face or the C face, each of which lends itself to slightly different properties for the graphene. This method is one of few that could be readily used in industry, as SiC is obtainable by businesses. It is costly, however, as is the energy cost required to conduct the sublimation [5].
 Following a thorough review of the basic principles surrounding graphene’s properties and synthesis, its use in prominent applications and industries can be discussed. One of the applications of using graphene that has shown promising results is water desalination. In conventional reverse osmosis, water is forced through a membrane at high pressure. As stated in the article, “the membrane lets the water molecules pass through but not the larger salt ions” [7]. Reverse osmosis is used at a large industrial level to desalinate water. However, the process does not work as well as it should because of membrane fouling and water metering. What engineers and scientists want to be able to do with graphene is create membranes or filters that are able to desalinate the water in large quantities and without the metering problem that conventional membranes suffer from. The membrane is also limited to the mechanism that drives the water across the membrane, diffusion. Some experts say that “increasing the membrane permeability by 3 fold, will decrease the energy consumption by 46%” [8]. The salt water is absorbed into the membrane and travel to the other side of the membrane as fresh water. This is where graphene is useful in making reverse osmosis a better filtration process.
Following a thorough review of the basic principles surrounding graphene’s properties and synthesis, its use in prominent applications and industries can be discussed. One of the applications of using graphene that has shown promising results is water desalination. In conventional reverse osmosis, water is forced through a membrane at high pressure. As stated in the article, “the membrane lets the water molecules pass through but not the larger salt ions” [7]. Reverse osmosis is used at a large industrial level to desalinate water. However, the process does not work as well as it should because of membrane fouling and water metering. What engineers and scientists want to be able to do with graphene is create membranes or filters that are able to desalinate the water in large quantities and without the metering problem that conventional membranes suffer from. The membrane is also limited to the mechanism that drives the water across the membrane, diffusion. Some experts say that “increasing the membrane permeability by 3 fold, will decrease the energy consumption by 46%” [8]. The salt water is absorbed into the membrane and travel to the other side of the membrane as fresh water. This is where graphene is useful in making reverse osmosis a better filtration process.
Fig. 5 – Graphene membrane used in reverse osmosis process [9]
Graphene would act as a filter wherein the only particles that would be able to pass through the structure of graphene are the smaller water particles. With graphene’s mechanical strength, size, and honeycomb shape, researchers speculate that with these filters it might be possible to filter up to “66 L per cm2-day-MPa with greater than 99% rejection rate” [7]. Comparing this to conventional reverse osmosis methods that average about 0.01 – 0.05 L cm2-day-MPa with unreliable rejection rates [7], it is clear to see why graphene is a super material that has a huge focus on large scale manufacturing. Figure 6 below shows the different flow rates for the desalination process [10]. The recommended sizes for the pores should be either 23.1 Å2 for hydrogenated pore, and hydroxylated pore 16.3 Å2 to be able to maximize water output through the graphene filter. Results also look promising for manufacturing large scale sheets of graphene. Researchers have been able to create a sheet of graphene that is 30 inches long [7, 10].

Fig. 6 – Salt Rejection compared to Water Permeability [10]
Graphene has many challenges to overcome before being commercially available for water desalination. The main problem is the size that the graphene is able to be produced at. As stated in the previous paragraph, the maximum size that any graphene sheet has been produced at is 30 in. With size being the main limiting factor for graphene production, the graphene works better than the membranes used for reverse osmosis now so even though it is smaller it is able to filter the seawater almost 600 times faster. It is also difficult to create the graphene sheets with the size holes that the filters would need to meet these demands. However, scientists are close to being able to increase the reliability of these pore sizes to correctly desalinate the water.
The experiment conducted by David Cohen-Tanugi and Jeffrey C. Grossman, wanted to compare the time for the particles to be filtered through different size pores of graphene with different pressures ranging from 1 MPa to 1500 MPa [10]. It was noticed that as the pressure increases, the flow rate of the water particles through the graphene sheet were linearly related. After the initial experiment with varying pressures, they focused on the 100 – 200 MPa range “in order to obtain well-converged statistics” [10]. With the increase in pressure, it was also possible that the salt ions would be able to pass through the graphene because of the effects that pressure has on larger cross-sectional area of the salt ions. Unsurprisingly, they found that the size of the pore affected the permeability of the water particles through the graphene sheet. When the pore size was about 62.1 Å2, the desalination was at the maximum before salt ions were able to pass through the graphene pores. Surrounding the pores were functional groups (-OH, COOH-, etc) that helped the water flow through the graphene with maximum stability in the graphene.
Simulations of these graphene sheets are conducted by many universities. Deepthi Konatham, Jing Yu, Tuan A. Ho, and Alberto Striolo are at the University of Oklahoma, and their research deals with the simulation of the water particles through the graphene sheets. In their experiment, they modeled a graphene membrane with different pore sizes and functional groups to determine the effects that each parameter has on graphene [11]. The simulations were carried out “at 300 K with a time step of 2 fs using the GROMACS 4.5.5 simulation package” [11]. Most of the experiments that involve graphene use this simulation software with a similar step and temperature. Since the pressure was not stated for this experiment, it is easy to assume that the pressure is around 100 – 200 MPa. After the experiment, the researchers plotted their data to visualize permeability values compared to the size of the pores.
For desalination through reverse osmosis, the process requires high pressures and could break the graphene filters, if there is excess pressure. At MIT, researchers conducted experiments that focus on the stress induced in the graphene when the pore size increases as well as higher pressures. Similar to bulk materials, they compared the stress at the membrane with the fracture stress of graphene sheets [8]. Later in the paper, they discuss determining the actual stress of the graphene membrane because at the size where the graphene fails behaves differently. Using this relation they were able to graph the stress of the membrane as a function of porosity. The maximum stress that the graphene exhibited was around 120 MPa with a porosity of 0 [8]. When water is introduced to the stress calculations, the graphene sheet experiences 35% more stress than the dry samples [8]. It is also stated that the future work should consider membrane fouling as that is a main cause for inefficiencies in the desalination of seawater [8].

Fig. 7 – Stress distribution in graphene with a = 0.5 nm [8]
3 Graphene Plasmonic Antennas
3.1 Introduction
In addition to the other varied and groundbreaking application fields graphene opens up, it has shown promise in solving two communication issues though graphene based antennas operating in the terahertz range. These antennas could be used at the nano scale for wireless communication between nano devices and the high frequency nature of their operation may provide a way to increase our communication bandwidth in the macro scale world. Making use of the electrical properties of graphene, a layer of surface plasmons are created that will react to electromagnetic waves in the terahertz range.
3.2 Communication Issues
The two issues, increasing our communication bandwidth and providing wireless communication between and out of nano devices hold back advances in transmitting the massive amounts of data our modern age can produce and consume as well as the near limitless possibilities of nano swarms and networks. These systems will enable the next level in reliable real time remote work, like remote surgeries will require massive bandwidth to deliver all the data a surgeon will need. Extra bandwidth will also help wireless systems connect more users from a single base station and reliable send massive amounts of data wirelessly.
3.2.1 Bandwidth
Currently most high speed wireless communication occurs in the gigahertz range, with 4g telephone systems operating between 1 and 2.7 GHz providing data at a max at 12 Mbps. Wi-Fi routers run in either 2.4 GHz or 5 GHz and can send data at a rate of 2.1 Gbps in the latest 802.11ac standard. To increase these speeds, higher frequencies with greater data densities will be required. Another reason to move high speed data communication out of the GHz range is the crowded spectrum space in that area, with WiFi routers, bluetooth communication, microwave ovens, and other devices all sending out em waves in that frequency range [12].
3.2.2 Nano device Communication
Potentially more important than raw speed, transmitting data between nano scale devices will open up many possibilities. Communication to and from nano devices can be obtained through indirect observation and manipulation. Observing quantum dots used in medical imaging is one basic example of rudimentary communication from a nano scale system. Other potential communication systems involve using molecules to transmit signals, similar to how hormones act as a communication system between cells. However, for high bandwidth data transmission from small nano computers with nano sensors, electromagnetic communication would expand the capabilities of these sensors. Especially with the addition of the ability to communicate within a swarm of deployed nano sensors. Unfortunately, there are issues with attempting to scale down antennas to a nano size.
One of the major issues comes from the frequency range available. The frequency, in MHz, that an antenna can pick up is equal to 300 divided by its length. A simple 1nm antenna would optimally receive a frequency of 3 million terahertz. This would be great for bandwidth. Unfortunately, as the frequency of an electromagnetic wave increases, the propagation distance of a wave generally decreases, with a few small exceptions at certain wavelengths. Therefore, more power is required to achieve a reasonable broadcast range. the power requirements for that range of communication would be larger than the current carrying capacity of the nano circuitry.
3.2.3 Current Antennas
For improved data rates, plasmonic antennas have been proposed and tested with different metals. By taking advantage of electromagnetic forces created at interaction of specific metals and a dielectric. These antennas can be small while still receiving the lower frequencies a larger antenna would collect [13]. Unfortunately for nanoscale applications, when other metals such as gold and silver are used the frequency is still in the infrared and visible light spectrum, which is 100 times greater than the frequency of the terahertz range, creating the same problem as the simple nanoscale wire antenna [14]. Graphene though, with its two dimensional geometry and unique conductive capabilities, shows great promise to solve this communication problem.
3.3 Graphene Nano Antenna
Like many applications of nanotechnology, complete transmission systems have not been created. In this case, a full receiver – transceiver systems have not been constructed. However, doped graphene configured in the form of finite length ribbons have been shown to propagate electromagnetic waves in the form of Surface Plasmon Polariton (SPP) waves at frequencies in the terahertz range. (Cite) This allows for transmission of information at a more usable rate than other nano scale antenna options.
3.3.1 Surface Plasma Polaritons
First observed in 1902 though anomalies from reflections in metal shavings, Surface Plasma Polaritons (SPP) are electromagnetic waves that exist in the region where a conductive material’s surface and a dielectric material meet. SPP waves themselves are characterized by alternating charges moving on the surface, carried along by the reinforcing em waves moving through the metal and dielectric in the area close to the interface.
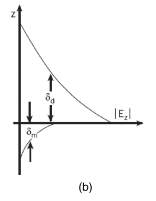 This interface region and its electromagnetic forces act a bit like a traditional wave guide antenna, containing and directing the incoming electromagnetic waves, propagating them though a properly tuned area with ease. Also, similar to wave guide antennas, the characteristics of the system must be designed to allow for specific wavelengths to travel through it. In operation, an external field moving through the dielectric will hit the conductive surface, creating a counter wave in the conductive material as well. The two electromagnetic waves will move though the conductive and dielectric materials, altered and contained by the short range attractive fields generated at the interface.
This interface region and its electromagnetic forces act a bit like a traditional wave guide antenna, containing and directing the incoming electromagnetic waves, propagating them though a properly tuned area with ease. Also, similar to wave guide antennas, the characteristics of the system must be designed to allow for specific wavelengths to travel through it. In operation, an external field moving through the dielectric will hit the conductive surface, creating a counter wave in the conductive material as well. The two electromagnetic waves will move though the conductive and dielectric materials, altered and contained by the short range attractive fields generated at the interface.
Fig. 8 –
 If the angle based on the frequency of the incoming wave meets a specific angle, these waves will harmonize and meet across the interface at regular intervals carrying along alternating charges on the surface of the metal. This angle, based on inherent light wavelengths of the metal and the dielectric and known as the critical angle, allows the waves within the dielectric and metal to reinforce each other with no loss of momentum. In this state, near infinite propagation of the waves will occur over the surface. Frequencies outside the critical angle will cause the waves will decay quickly [13]. The incoming frequency will determine the angle of incidence at the interface. Therefore, the material properties involved in the system set the frequency that will be favored by the antenna design. As noted earlier, using traditional metals requires a frequency so high that is not optimal for nano or long range communication. Also, since the critical angle is property of the metal and only certain metals work, there is little ability to tune the system for specific frequencies.
If the angle based on the frequency of the incoming wave meets a specific angle, these waves will harmonize and meet across the interface at regular intervals carrying along alternating charges on the surface of the metal. This angle, based on inherent light wavelengths of the metal and the dielectric and known as the critical angle, allows the waves within the dielectric and metal to reinforce each other with no loss of momentum. In this state, near infinite propagation of the waves will occur over the surface. Frequencies outside the critical angle will cause the waves will decay quickly [13]. The incoming frequency will determine the angle of incidence at the interface. Therefore, the material properties involved in the system set the frequency that will be favored by the antenna design. As noted earlier, using traditional metals requires a frequency so high that is not optimal for nano or long range communication. Also, since the critical angle is property of the metal and only certain metals work, there is little ability to tune the system for specific frequencies.
Fig. 9 –
3.3.2 Graphene Antenna
Graphene’s structure and unique electrical properties provide a very tunable platform for SPP antenna applications. By adding small impurities through doping, the graphene’s electrical properties can be altered and the critical angle tuned for many different frequencies within the terahertz range.
The shape of the graphene affects how the material reacts to the frequency. In an infinite plane, conductivity of the graphene does not change based on the frequency, providing little use as a plasmonic antenna. The ribbon shape on the other hand, confines the electrons and restricts their movement laterally [15]. The ribbons can be in either the armchair or zigzag configuration with different effects, but both provide suitable antenna for use [14]. This configuration and the ability to alter conductivity though doping allow for graphene’s use as a highly tunable electromagnetically conductive antenna. 
Fig. 10 –
Like a traditional SPP antenna, the conductive ribbon is attached to a dielectric layer. That layer can also contain the doping atoms that can work its way into the hexagonal graphene structure. With graphene as the conductor, the material is too thin to support waves running though it, like the metal SPP antennas. Graphene, does however conduct electricity in unique ways and when an electromagnetic wave excites the electrons in the graphene nano ribbons they will oscillate around dynamically in response to the wave [16].
Fig. 11 –
These dynamic complex waves reinforce the dielectric waves and create the SPP waves of electric charges at the interface between the graphene and the dielectric. Unlike the metal plasmonic antennas, the waves in the graphene are dependent on the energy bands that are allowed by the graphene structure. Zigzag or Armchair configurations that run on the edge of the long sides of the nano ribbon dictate which bands will be accessible to the electrons in the doped graphene lattice.
3.3.3 The Future
Currently researchers are modeling these antennas to predict their properties in different configurations to aid in fabrication of full communication systems. One promising model, shows how effective they will be in an array pattern that will be useful for the large scale wireless communication of cell phones and wireless routers [17]. Some different input and output methods have been proposed, with many challenges still ahead for creating a full transceiver system. However, with the design of a graphene antenna that works in a usable and practical frequency range developed over the last couple of years, interest is growing in designing the other equipment to make a complete communication system that works at the nano scale.
Another very interesting way in which this exciting material can be used is in electron storage in either batteries or capacitors. Graphene is a very efficient electrical and thermal conductor, lightweight, chemically inert with an extremely high surface area per unit volume. Advancement in these fields continues at a breakneck pace with new developments all the time. ongoing but will need a great deal of research and development before wide spread commercialization will be seen in the market [18]. Besides the initial procurement of the technology required, there will need to be a transition because there has already been considerable resources laid out for existing technology and so prices must come down and new contracts established before the switch can occur. The transition to a lithium/graphene battery hybrid would be much easier to facilitate given the current allocation of existing resources. This transition may be furthered by the problem that rare earth metals are indeed rare and with demand increasing so too is price [19].
Lithium ion batteries can supply and store energy over long periods of time with a very high charging cycle life [20]. Great advancements have been made on this front many in the form of anode and cathode improvements. Anode materials with a much higher capacity such as tin and silicon transition metal oxides. One such material with much promise is Fe3O4 because it is of great abundance, read low cost, and high capacity. The introduction of carbon with carbon coated nano-spindles of Fe3O4, Fe3O4-based Cu nano-architecture, magnetic/carbon core-shell nano-rods, and iron oxide based nanotube arrays have all been used as a way aimed at improving the electrochemical performance of iron oxides. Although many of these high capacity electrochemical materials are currently followed with problems of rapid capacity fading because of a pulverization during the load cycle which often leads to a breakdown of electrical anode connections and current collectors.
Though carbon nanotubes exhibit some problems allowing the functional motion of the electrolyte volume expansion, also the large surface are of the nanotubes increases the risk of secondary surface reactions for electrolyte decomposition [21]. One such solution to this problem has been suggested in the form of graphene nano- sheets because of the porous texture of this material [22]. When this is done in layered sheets it provides a flexibility the the nano-tubes cannot provide because of the lack of rigid connection between the sheets. When combined with its high electrical conductivity this flexible and porous is used to confine but still shows a substantial buffering capability to reduce the fore mentioned pulverization. It is because of these abilities that graphene has been looked at as a desirable material for used in lithium ion batteries. However due to the problems of single layer graphene production, because of aggregation, it is difficult to demonstrate these superior properties of graphene.
Rice University has made one very useful contribution to the concept of integrating graphene and CNT’s (carbon fiber nanotubes) into lithium ion batteries. In 2012 researchers at Rice University used CNT’s to store lithium uniquely in the anode using the three dimensional surface. This anode further approaches the theoretical maximum for the storage of lithium in the space. This approach also resists forming dendrites or “mossy” deposited that harm or damage the battery. While lithium ion batteries are much better than traditional nickel cadmium batteries of the past in both energy storage and operational life, more is always desired. For years scientists have tried to replace lithium-ion batteries with more efficient and powerful lithium
metal batteries but the formation of dendrites have limited this progress [23]. The lithium metal

Fig. 12 – [23]
batteries would last longer and charge faster than lithium-ion batteries. Dendrites are deposited of lithium that can grow into the electrolyte of the battery. The danger is if they are able to bridge the anode and cathode thus short circuiting the battery causing it to fail. The failure may even result in a fire or explosion. It was found by chemist James Tour that, with this new approach, when the batteries are charging the lithium metal very evenly coats the carbon surface. These highly conductive carbon hybrids of CNTs and graphene covalently bond and are coated with the lithium metal trading energy capacity for increased safety. A great deal of research in this field is being done to further exploit the potential of graphene fir use in battery systems. The American Chemical society journal Nano shows the depth of the vast research committed to this endeavor. Using CNTs, with their high surface area and low density, allows for lithium-ion particles to move back and forth when charging and discharging the battery. More than that because of the even distribution of the lithium metal the formation of problematic dendrites is limited.

 Fig. 13 – [23]
Fig. 13 – [23]
Fig. 14 – [23]
While this is a promising path toward improving lithium battery technology much of the technology is focused on the anode but not the cathode. There is good reason for this problem, mainly because the anode research has had much done and the cathode is more difficult. Dr. Abdul-Rahman O. Raji at the University of Cambridge has been doing research with lithium cobalt oxide in the cathode for use in fuel cells. This has shown promise because of it showing a very flat voltage profile with no dendrite growth. This entire field is growing and ever evolving but with more research and development much more advancement in this exciting field should be expected.
An area of concern in all of this is environmental impact. While the use of electrical power instead of fossil fuels is seem as a great alternative the subject is simple more complicated than that. From the department of energy, our power comes from the following sources as follows: natural gas 34%, coal 30%, nuclear 20% petroleum 1%, and renewables 15%. These renewables can be broken up as follows: hydro 6.5%, wind 5.6%, biomass 1.5%, solar 0.9% and geothermal 0.4%. This is also further complicated by the pollution caused by the mining of the rare earth metals and other elements required for battery production. Besides the pollution caused there is the human cost. An example is that while a large portion of the world coal comes from Congo it is loosely regulated and often that cost is shouldered by the poor workers in this impoverished nation [24]. A possible answer to this is IN the form of graphene super capacitors.
Beyond batteries another form of electric charge storage is the capacitor. Th form that has more recently been under more focus is specifically the super-capacitor or EDLC (electric double-layer capacitor). The biggest difference between a regular capacitor and a super-capacitor is simply the charge that can be stored. New super-capacitors can rival lithium-ion batteries [25]. Since batteries store energy in a chemical reaction and capacitors do so in an electric field, capacitors do not suffer from the degradation or the long charging times that batteries do.
In capacitors, what dictates the amount of electricity that can be stores is the surface area of the conductive material. Graphene have a very high surface area and is very conductive. In the past, typically, super-capacitors have been able to store about 150 Farads/gram but the theoretical limit for graphene given it high surface area is about 550 Farads/gram [26]. Due to it varied and versatile material properties graphene super-capacitors would inherit good mechanical strength and elasticity with very large surface area for charge storage and not suffer from thermal problems of batteries. Graphene based capacitors are currently a reality but super-capacitors have are currently very expensive to produce and while the promises of this technology is promising more development is needed. With all nano technology much of the problems associated with these technologies is bulk production and scalability. The social or societal impacts cannot be over stressed, one of the greatest challenges to faced by human kind is energy production and the storage of said energy. As there is an immense interest in this technology and because of the potential profits associated with being the first to develop and patent it the current state of development on a commercial scale is closely guarded. So because of that information is sparse to say the least. Though, to show the real and intense interest of progression on this front, Lamborghini is currently working with M.I.T to develop an electric supercar powered by super-capacitors. The companies reasoning for choosing super-capacitors is that besides shorter charging times, a battery simple could not provide the power that a supercar would demand. Shown below, the Lamborghini Terzo Millennio concept [27].

Fig. 15 – [27]

Fig. 16 – [28]
References
[1] “This Month in Physics History: October 22, 2004: Discovery of Graphene.” American Physical Society. N.p., n.d. Web. 09 Sept. 2017.
[2] Class for Physics of the Royal Swedish Academy of Sciences. Scientific Background on the Nobel Prize in Physics 2010: Graphene. N.p.: n.p., n.d. PDF.
[3] Novoselov, Konstantin S., and A. K. Geim. “The rise of graphene.” Nat. Mater 6 (2007): 183-191.
[4] Warner, Jamie H., et al. Graphene : Fundamentals and Emergent Applications. vol. 1st ed, Elsevier, 2013. EBSCOhost,
search.ebscohost.com/login.aspx?direct=true&db=nlebk&AN=486005&site=ehost-live.
[5] Edwards, Rebecca S., and Karl S. Coleman. “Graphene Synthesis: Relationship to Applications.” ChemInform, vol. 44, no. 13, 2013, doi:10.1002/chin.201313233.
[6] Abbasi, Elham, et al. “Graphene: Synthesis, Bio-Applications, and Properties.” Artificial Cells, Nanomedicine & Biotechnology, vol. 44, no. 1, Feb. 2016, pp. 150-156. EBSCOhost, doi:10.3109/21691401.2014.927880.
[7] Wang, Evelyn N., and Rohit Karnik. “Water desalination: Graphene cleans up water.” Nature nanotechnology 7.9 (2012): 552-554.
[8] Cohen-Tanugi, David, and Jeffrey C. Grossman. “Mechanical strength of nanoporous graphene as a desalination membrane.” Nano letters 14.11 (2014): 6171-6178.
[9] “Desalination is Graphene latest miracle.” WordlessTech, 5 July 2012, wordlesstech.com/desalination-across-nanoporous-graphene/.
[10] Cohen-Tanugi, David, and Jeffrey C. Grossman. “Water desalination across nanoporous graphene.” Nano letters 12.7 (2012): 3602-3608.
[11] Konatham, Deepthi, et al. “Simulation insights for graphene-based water desalination membranes.” Langmuir 29.38 (2013): 11884-11897.
[12] Akyildiz, Ian F., Josep Miquel Jornet, and Chong Han. “Terahertz band: Next frontier for wireless communications.” Physical Communication 12 (2014): 16-32.
[13] Zhang, Junxi, Lide Zhang, and Wei Xu. “Surface plasmon polaritons: physics and applications.” Journal of Physics D: Applied Physics 45.11 (2012): 113001.
[14] “Graphene-Based Plasmonic Nano-Antenna for Terahertz Band Communication in Nanonetworks.” IEEE Journal on Selected Areas in Communications, Selected Areas in Communications, IEEE Journal On, IEEE J. Select. Areas Commun, no. 12, 2013, p. 685. EBSCOhost, doi:10.1109/JSAC.2013.SUP2.1213001.
[15] Dorfmuller, Jens, et al. “Plasmonic nanowire antennas: experiment, simulation, and theory.” Nano letters 10.9 (2010): 3596-3603.
[16] Toon, John. “Graphene-Based Nano-Antennas May Enable Networks of Tiny Machines.” Georgia Tech News Front Page, Georgia Institute of Technology, 11 Dec. 2013, www.news.gatech.edu/2013/12/11/graphene-based-nano-antennas-may-enable-networks-tiny-machines.
[17] Zakrajsek, Luke, et al. “Design of graphene-based plasmonic nano-antenna arrays in the presence of mutual coupling.” Antennas and Propagation (EUCAP), 2017 11th European Conference on. IEEE, 2017.
[18] Inc., Sponsored by Cheap Tubes. “Understanding Graphene Batteries.” AZoNano.com, 3 Aug. 2017, www.azonano.com/article.aspx?ArticleID=4326#1.
[19] Motavalli, Jim. “Forget Lithium — Its Rare Earth Minerals That Are in Short Supply for EVs.” CBS News, CBS Interactive, 19 June 2010, www.cbsnews.com/news/forget-lithium-its-rare-earth-minerals-that-are-in-short-supply-for-evs/.
[20] Zhou, Guangmin, et al. “Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries.” Chemistry of Materials 22.18 (2010): 5306-5313.
[21] Zhang, Wei‐Ming, et al. “Carbon Coated Fe3O4 Nanospindles as a Superior Anode Material for Lithium‐Ion Batteries.” Advanced Functional Materials 18.24 (2008): 3941-3946.
[22] Lv, Wei, et al. “Low-temperature exfoliated graphenes: vacuum-promoted exfoliation and electrochemical energy storage.” ACS nano 3.11 (2009): 3730-3736.
[23] “Graphene-Nanotube hybrid boosts lithium metal batteries.” Phys.org – News and Articles on Science and Technology, phys.org/news/2017-05-graphene-nanotube-hybrid-boosts-lithium-metal.html.
[24] Frankel, Todd C. “The Cobalt Pipeline.” Washington Post, 30 Sept. 2016, www.washingtonpost.com/graphics/business/batteries/congo-cobalt-mining-for-lithium-ion-battery/.
[25] “Graphene Supercapacitors: Introduction and News.” Graphene-Info, www.graphene-info.com/graphene-supercapacitors.
[26] Fuente, Jesus de La. “Graphene Supercapacitors – What Are They?” Graphenea, www.graphenea.com/pages/graphene-supercapacitors.
[27] O’Kane, Sean. “The Lamborghini Terzo Millennio concept is a lightning strike from the future.” The Verge, The Verge, 6 Nov. 2017, www.theverge.com/transportation/2017/11/6/16613998/lamborghini-terzo-millennio-electric-concept-car-photos.
[28] “Graphene batteries: Introduction and Market News.” Graphene batteries: introduction and market status | Graphene-Info, www.graphene-info.com/graphene-batteries.
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more