Introduction
Skeletal muscles have specific characteristics such as irritability, conductibility, contractility, and adaptability (Enoke, 2015). These characteristics give skeletal muscles the ability to; respond to stimuli, emanate excitation waves, change the length of muscle fibres (stretch), and grow and regenerate to a variability limit (Enoke, 2015). In response to the stimulus, due to Henneman’s size principle, the smallest motor unit of skeletal muscles, is first to activate (L. Brent, personal communication, October 23rd, 2017). A motor unit is composed of alpha motor neurons (α-MN) and all the muscle fibres it innervates (L. Brent, personal communication, October 23rd, 2017). There are 3 types of motor units that gradually increase in axon size and velocity that are as follows; slow (smallest/slowest), intermediate and fast twitch (largest/fastest) (L. Brent, personal communication, October 23rd, 2017). The excitation or action potential (AP) of the motor unit causes depolarization and repolarization along the α-MN and the sarcolemma due to its semi-permeable membrane (Enoke, 2015). These myoelectrical signals can be recorded as Electromyography (EMG) (L. Brent, personal communication, October 23rd, 2017). Contraction of the muscle fibres innervated by the motor units can produce force (in newtons/ N) when it is triggered by the AP (Enoke, 2015). This is because, an AP causes an excitation-contraction coupling (E-C coupling) allowing the release of calcium ions (Ca²+) from the sarcoplasmic reticulum to flow into the inter-cellular space (L. Brent, personal communication, October 23rd, 2017). Ca2+ ions bind to the contractile apparatus of the muscle to shorten and ultimately produce force (Enoke, 2015). Biomechanical force is the sum of external torque from active muscles (Maton & Bouisset, 1977).
The conductance of velocity of the motor units is recorded from a fixed point with units voltage or millivoltage (EMG). Typically, the velocity of motor units is constant along the path of muscle fibres (Gygi & Moschytz, 2002). Therefore, any high frequencies of EMG activity get cut out by a low-pass filter. Low-pass filtering smooths out the data as it passes through and preserves lower voltage EMG (L. Brent, personal communication, October 25th, 2017).
Excitation of the motor units correlates to the contraction of the muscle fibres within the muscle of interest. Therefore, as muscle force increases, there is an increase in EMG activity from spatial and temporal recruitment. Spatial recruitment means more populous of motor units involved (L. Brent, personal communication, October 25th, 2017). Whereas temporal recruitment increases the frequency of the rate at which active motor units are being fired (L. Brent, personal communication, October 25th, 2017).
The main purpose of this lab is to collect and record EMG activity to its corresponding force production in an isometric contraction. The two upper limb muscles that are examined are the heterogenous biceps brachii (BB), which is responsible for flexion at the elbow when the hand is in supination, and the brachioradialis (BR), which also contributes to flexion at the elbow when the hand is in a neutral position. This allowed us to analyse and understand the hypothetical linear EMG/Force relationship (L. Brent, personal communication, October 23rd, 2017). Therefore, we hypothesize that force and EMG activity will have a positive relationship due to an increase in spatial and temporal summation to increase force. We do not expect to obtain a linear relationship due to factors of synergist muscles to BB, EMG recording of motor units in only superficial muscle, and heterogenous BB that may give way to a non-linear force/EMG relationship.
Methods
One healthy 20-year-old male, with height of 5’7 feet, weight of 66.8 kg, and high active status participated in the study.
EMG can be recorded on superficial muscles of the body through electrodes placed on surfaced skin of the participant. In this lab we recorded EMG through two LP511 EMG amplifiers and with the use of “Logger Pro”. Since the signal of EMG is very small, and surface electrodes collect environmental noise very well. We used a bipolar configuration (2 LP511 EMG amplifiers) to amplify what is different and cut out any noise frequency that was common between them. Similarly, to minimalize interference the surface skin where the electrodes are placed was cleaned with alcohol.
The participant had 2 electrodes placed lengthwise on the bellies for both BB and BR. These coupling electrodes had no space in between them. Two additional electrodes were placed on a biological ground site for each muscle on the participant. This ground site is also known as a reference site and is important for subtracting out biological noise that is unrelated electrical tissue and not muscle (L. Brent, personal communication, October 25th, 2017). One was placed on the wrist at the landmark of stylist of the ulna and another on the elbow at the landmark of the medial epicondyle of the humerus. There were 3 leads for each muscle, green leads were attach to the ground sites and a negative and positive lead to each muscle. Leads were then attached to electrodes and have an input on the LP511 EMG amplifier.
The LP511 EMG amplifier settings were set as follows, CAL to use, (-6dB) low frequency filter to 10 Hz, (-6dB) high frequency filter to 1kHz, amplification to 5k and line filter and out. A Lab Quest Mini DAQ was used to provide channels for the force plate, and the two EMG leads. The hardware for the amplifier was setup manually in Logger Pro, whereas the force plate automatically synced itself. We had set each sensor channel individual to ‘Raw Voltage +/- 10V’. For our data collection parameters, we used a sampling rate of 2000 sample/second and set the duration to 5 seconds. In data triggering, we had selected ‘Triggering’ and ‘increasing’, and changed the ‘N box’ to a value of 10 and ‘Samples’ to 1000. We had then zeroed the channel containing the force plate only, using the ‘zero sensor calibration’ dialog box.
The participant sat in a chair facing the force plate. The participant’s starting position was a 90 degrees flexion at the elbow. Collection of the participant’s unprocessed maximum voluntary contraction (MVC) for BB and BR was then performed. Two trials of each muscle were recorded. When BB was recorded the participant positioned their hand in supination against the force plate and when BR was recorded the participant positioned their hand in a neutral position against the force plate. Prior to each contraction the participant had a relaxed arm and waited 5 seconds once ‘collect’ was clicked. Waiting these 5 seconds is important for collecting the background noise data. Then during each contraction, the participant slowly accelerated up to MVC against the force plate, held MVC for a few seconds and slowly declined back down.
Our participant then performed 3 submaximal contractions including, mild, medium and strong. These contractions were aimed to be about 15-25%, 45-55% and 75-85% of MVC force of BB, respectively. More specifically, our participant aimed for 20%, 50%, and 75% of 261.9N. All submaximal trials were performed in the same position and had the same procedure as MVC of BB. All submaximal trials were then processed in both muscles including, removal of bias, rectification and low-pass filtered at 10 Hz. Removing the bias is essentially zeroing the data or shifting resting EMG upwards or downwards to around 0 (L. Brent, personal communication, November, 2017). This is manually done by subtracting the current baseline value from the mean of the entire dataset (L. Brent, personal communication, October 27th, 2017). However, this can be done automatically in Logger Pro, through creating a ‘New Calculated Column’. The average level of EMG signal is denoted by the EMG amplitude but since EMG activity oscillates around zero, we would yield an average amplitude of about zero (L. Brent, personal communication, October 27th, 2017). Therefore, to obtain a value that is non-zero we must rectify the data. Rectification made all our values absolute (positive) (L. Brent, personal communication, October 27th, 2017). To rectify the data in Logger Pro, a ‘New Calculated Column’ is needed again. To isolate the EMG signal from environmental noise or artifacts, the EMG data will be low-pass filtered at 10 Hz (De Luca, Donald Gilmore, Kuznetsov, & Roy, 2010). This filters out all the high frequency data. This was similarly done in Logger Pro as rectification. In addition, the same data set was also processed with removal of bias, rectification, and low-pass filtered at 4 Hz. Data from all processed EMG submaximal contractions were then normalized. Normalizing EMG to a percent of its MVC is beneficial due to it being represented in relative terms allowed us to compare EMG between muscles (BB vs. BR), muscles from other participants, and throughout time of the same muscle (L. Brent, personal communication, October 27th, 2017). Normalizing was completed in Logger Pro with ‘New Calculated Column’.
The average EMG amplitude was collected from the BB submaximal graphs. Average EMG amplitude during a contraction has a duration of about 200ms (L. Brent, personal communication, October 27th, 2017). This time window was placed in an alignment on both force and its corresponding EMG graph.
Results
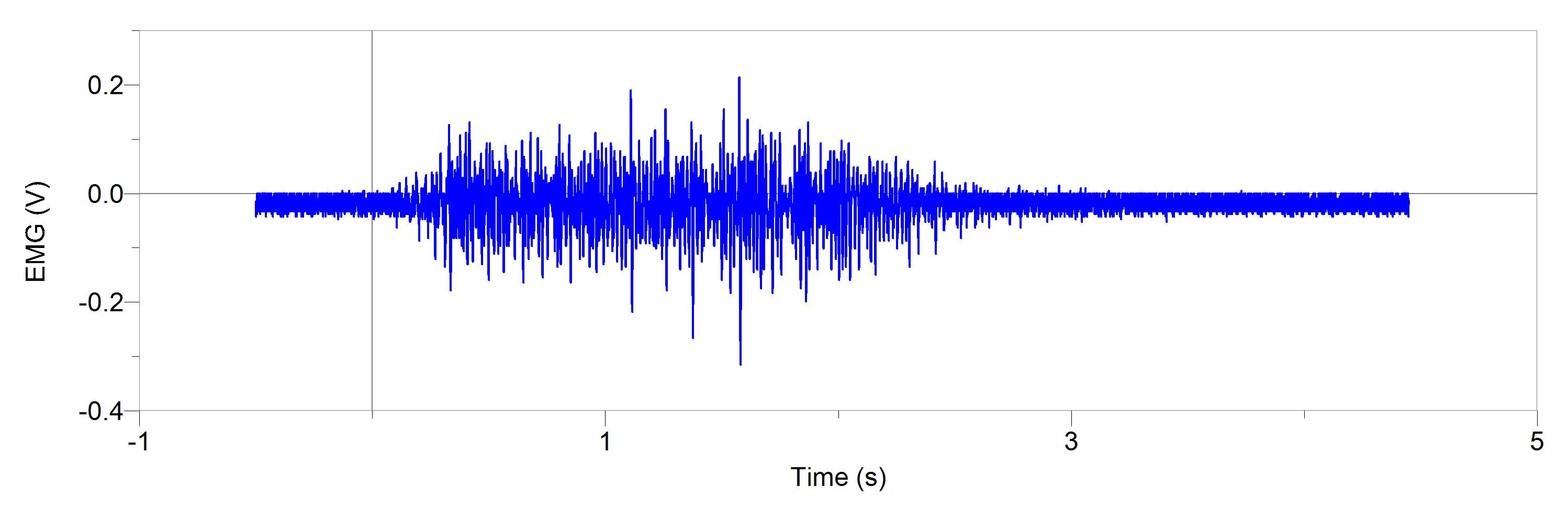
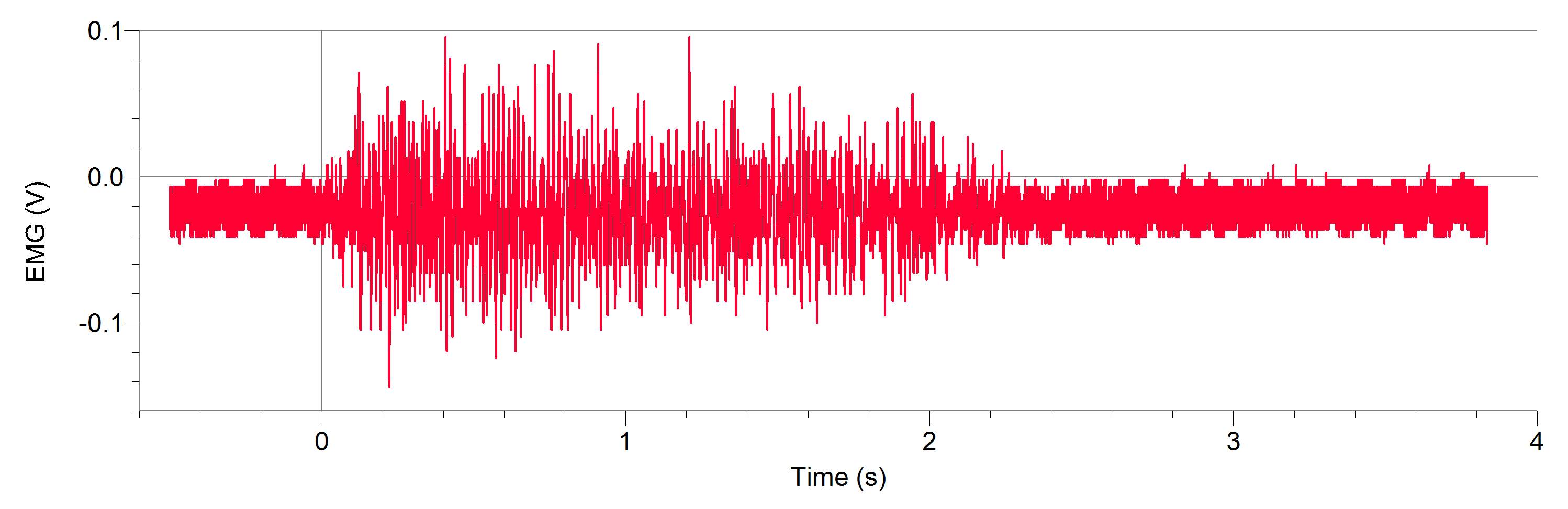
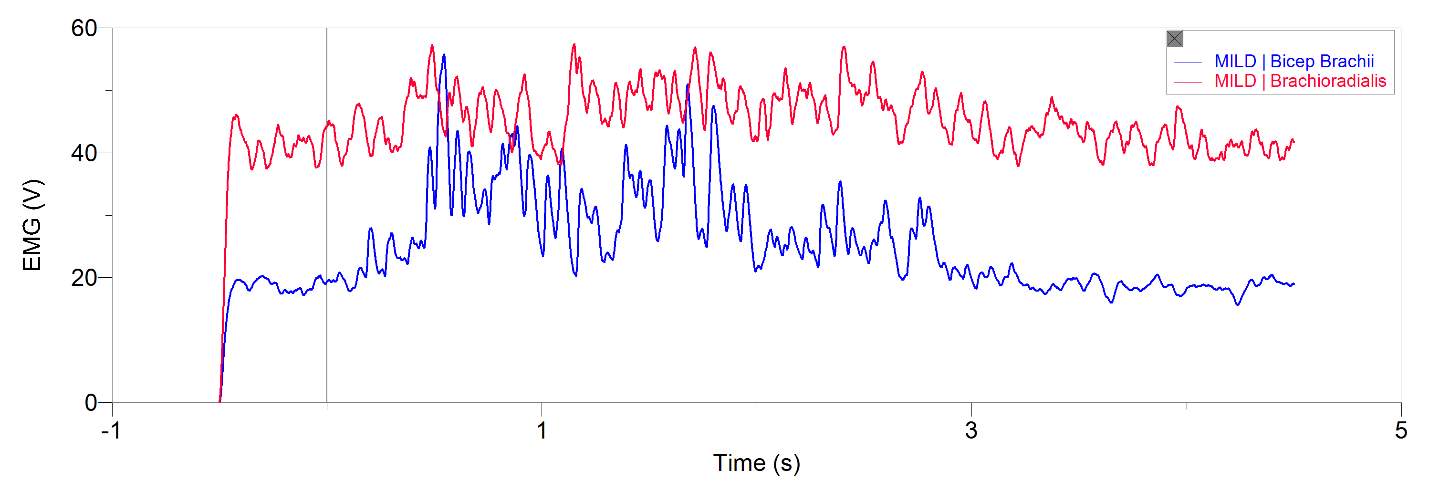
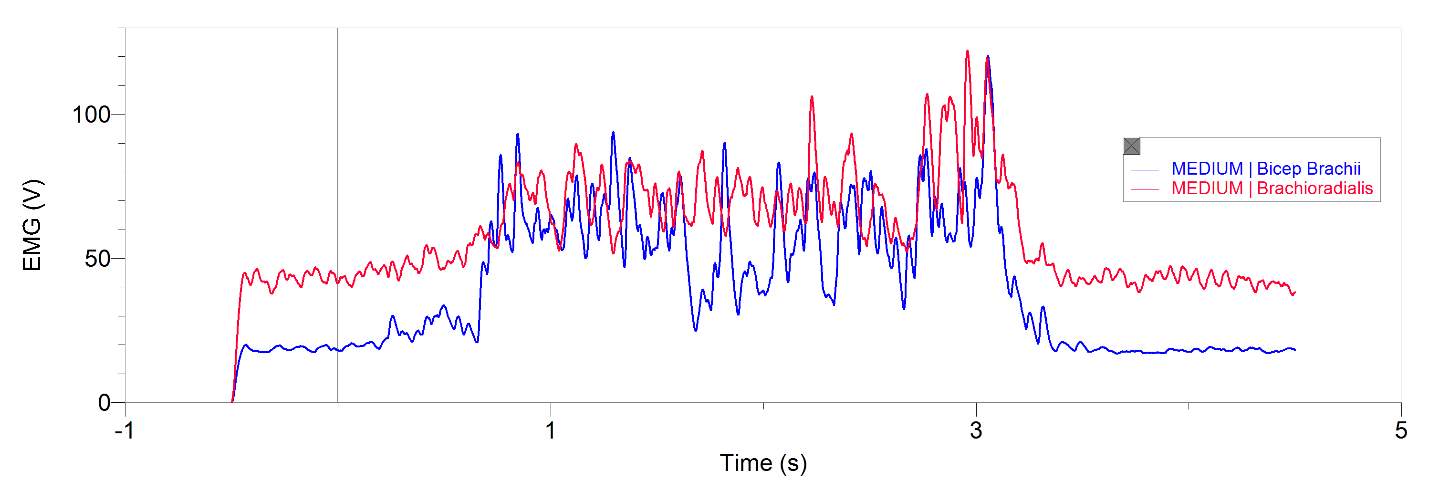
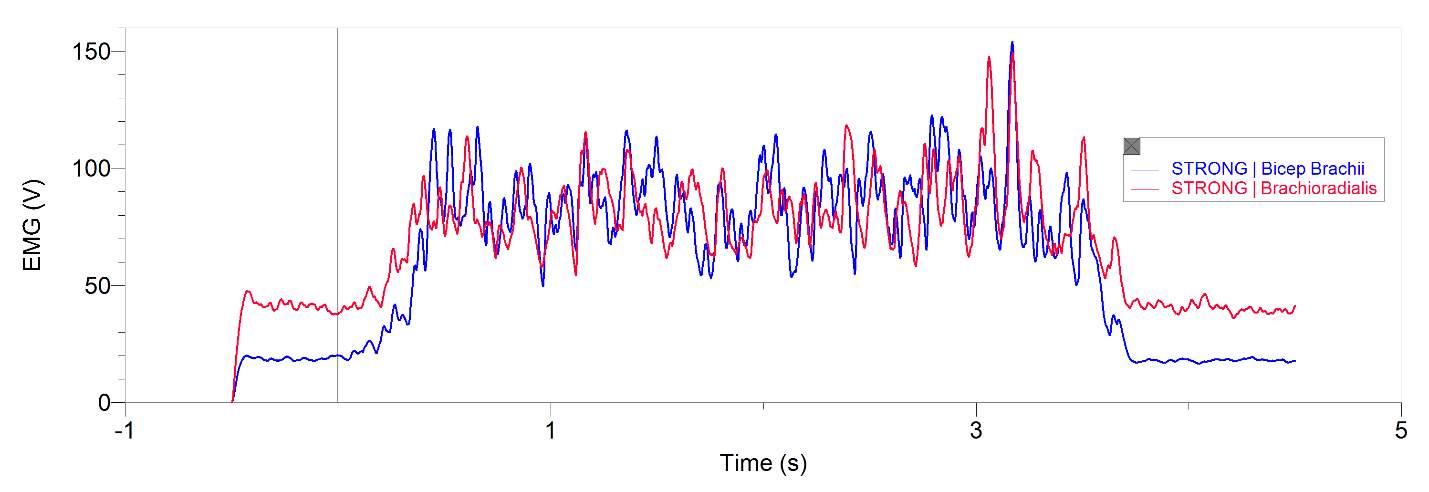
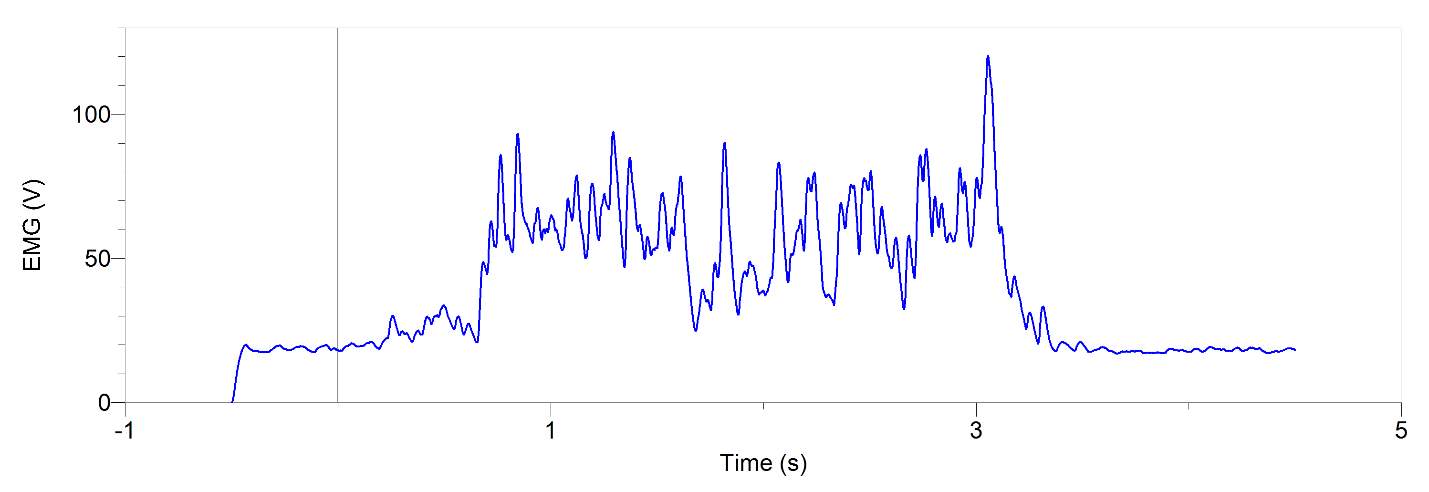
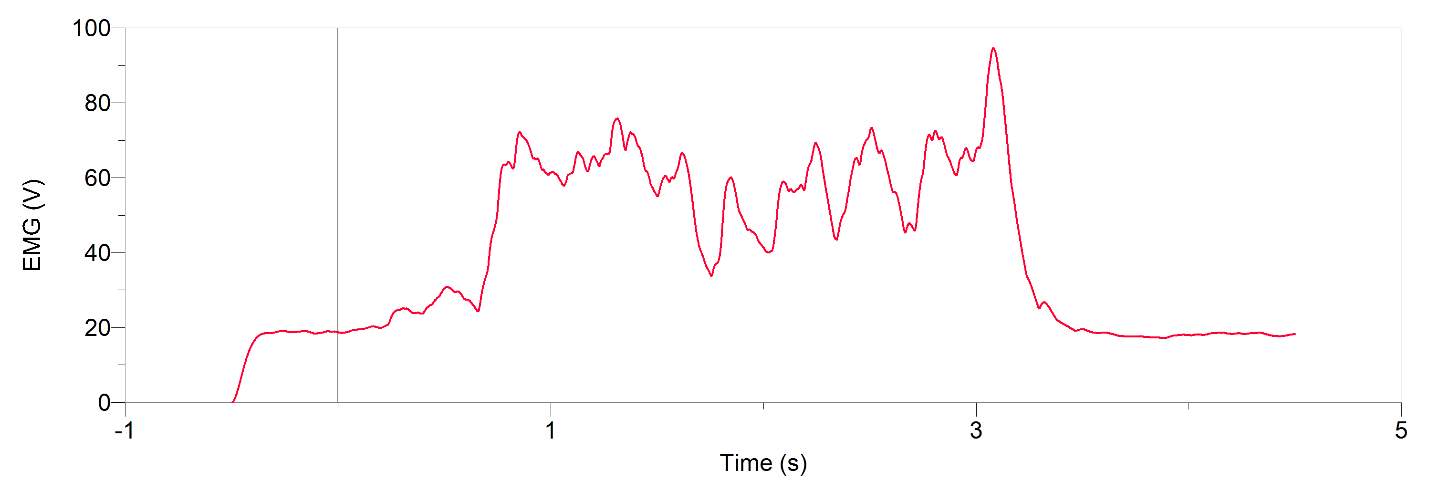
Figures 1 and 2, show our participant’s MVC of unprocessed EMG activity in the bicep brachii and brachioradialis, respectively. The participant achieved MVC of 261.9N in BB and 241.6N in BR. EMG amplitude measured in voltage in both muscles oscillates around zero for a contraction duration of 2.5 seconds for BB (figure 1) and 2 seconds for BR (figure 2). Before and after contraction there is reference EMG the inactive muscles as it stays steady at zero amplitude. In figures 3,4 and 5, a processed EMG activity at mild, medium and strong submaximal power outputs (or force) respectively. These EMG were processed with, bias removal, rectification, normalized, and low-pass filtered at 10 Hz. The BB EMG amplitude increased as a percentage of MVC from mild to medium and from medium to strong in both BB and BR. These values include for BB mild to medium of about 25% to 48% and from medium to strong of about 49% to 85%. Figure 6 and 7 are from the same trial as they are both representations of the EMG activity in the bicep brachii. EMG was processed with removal of all bias’s, rectified, and normalized. However, they differ in the low-pass filter being used. In Figure 6 a low-pass filter at 10 Hz was used. Whereas in Figure 7 a low-pass filter at 4 Hz was used.
The relationship between submaximal power outputs of mild, medium and strong and EMG activity was plotted in Figure 8. This plotted data only represents EMG activity in the bicep brachii and does not account for any other muscles facilitating the force. At the lowest force output of 60.9N (mild trial), and its equivalence of 23% of participant’s MVC, corresponded to EMG activity of 25% of maximal recorded EMG activity. At the highest force output of 187.0N (strong trial) or 72% of MVC, had a corresponding percentage of maximum EMG activity of 85%. Table 2 represents the data from figure 8. Table 2 shows the values of submaximal force respectfully to a percentage of processed EMG maximum. Processed EMG in table 2 includes, removal of any bias’s, rectified, normalized, and low-pass filtered at 4 Hz.
EMG (V)
Figure 1: A 20-year-old male participant’s unprocessed EMG activity in volts at MVC in bicep brachii for approximately 2.5 seconds
EMG (V)
Figure 2: A 20-year-old male participants unprocessed EMG activity in volts at MVC in brachioradialis for approximately 2 seconds
Percent of maximum EMG (V)
Figure 3: 20-year-old male’s bicep brachii and brachioradialis processed EMG activity in volts at a mild submaximal effort for 5 seconds. The data has had any bias removed, rectified, normalized and low-pass filtered at 10 Hz
Percent of maximum EMG (V) (mV)
Figure 4: 20-year-old male’s bicep brachii and brachioradialis processed EMG activity in volts at a medium submaximal effort for 5 seconds. The data has had any bias removed, rectified, normalized and low-pass filtered at 10 Hz
Percent of maximum EMG (V)
Figure 5: 20-year-old male’s bicep brachii and brachioradialis processed EMG activity in volts at a strong submaximal effort for 5 seconds. The data has had any bias removed, rectified, normalized and low-pass filtered at 10 Hz
Percent of maximum EMG (V)
Figure 6: 20-year-old male’s bicep brachii processed EMG activity in volts at a medium submaximal effort for 5 seconds. The data has had any bias removed, rectified, normalized and low-pass filtered at 10 Hz
Percent of maximum EMG (V)
Figure 7: 20-year-old male’s bicep brachii processed EMG activity in volts at a medium submaximal effort for 5 seconds. The data has had any bias removed, rectified, normalized and low-pass filtered at 4 Hz
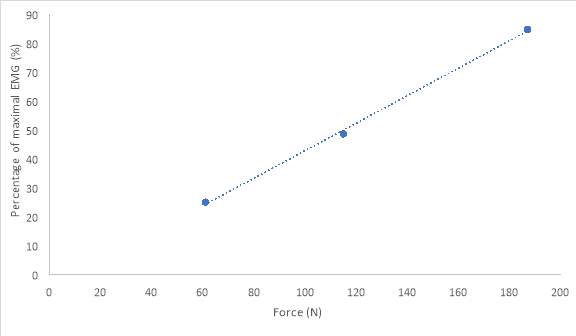
Figure 8: 20-year-old male’s relationship between force (N) at each submaximal trials of mild, medium, and strong to a percentage of maximal processed EMG activity in bicep brachii. The data has had any bias removed, rectified, normalized and low-pass filtered at 4 Hz
Table 1: MVC achieved by 20-year-old male participant, with corresponding unprocessed EMG activity in both the bicep brachii and brachioradialis
| MVC TRIAL |
BICEP BRACHII | BRACHIODRADIALIS | ||
| EMG (V) | FORCE (N) | EMG (V) | FORCE (N) | |
| 1 | 0.06329 | 261.2 | 0.02269 | 236.2 |
| 2 | 0.04865 | 245.2 | 0.02379 | 241.6 |
| AVERAGE | 0.05597 | 0.02324 | ||
Table 2: Submaximal forces achieved by 20-year-old male participant, with corresponding percentage of maximal processed EMG activity, in the bicep brachii only.The data has had any bias removed, rectified, normalized and low-pass filtered at 4 Hz
| SUBMAX TRIAL | Force (N) | EMG (%) |
| Mild | 60.9 | 25.09 |
| Medium | 114.9 | 48.72 |
| Strong | 187.0 | 84.84 |
Discussion
In this study the main objective was to generate force onto a force plate and record the corresponding EMG. The main action performed by the participant was an isometric contraction, at a position of flexion at the elbow at 90 degrees and supinated. As mention previously force is the sum of all the contracting muscle contributing to the force for that action (Maton & Bouisset, 1977). When EMG was recorded from our participant’s BB in correspondence to force generated, the force generated was a contribution of multiple muscles and not just the BB alone. Therefore, there are two synergist muscles to BB (Maton & Bouisset, 1977). These muscles include BR and brachialis (Maton & Bouisset, 1977). One of the actions of BR is flexion of the forearm at the elbow joint. BR flexion is optimal when the hand is in a neutral position, but is not limited to this position. Similarly, brachialis is also responsible for flexion at the elbow in all positions of the hand (supination, pronation, neutral). BR and brachialis are active during contraction and will have input in the force being generated. Therefore, synergist muscles to BB will contribute to force but not contribute to any EMG activity in the BB (Maton & Bouisset, 1977). Ultimately, this would affect the relationship between force and EMG in the BB. As a result, this decreases the linearity, since force is increasing at a greater amount then there is EMG activity in the BB. In addition, EMG activity may also not be entirely accurate due to the placement of electrodes. In this study we used surface electrodes attached to the skin. This may result in the collection of superficial motor units activity, instead of collecting motor unit activity from the entire muscle. This could alter the linearity between force and EMG since the entire force generated was collected and only a fraction of EMG may have been collected. This can be see in figure 8 through the line that best fits for graph of force/EMG as is it close but is not perfectly linear. However, there is still a definite positive correlation between EMG and force.
Using the data from Table 2, we can express the force generated as a percentage of MVC that corresponded to Figure 1. Maximal force generated by the participant was 261.2 N. Therefore, at our participant’s submaximal trials of mild, medium and strong, the participant obtained 23%, 44% and 72% respectively. Our participant’s medium and strong trials had not reached expected values. These values were ideally to lie around 45-55% and 75-85% respectively. Our participant may have achieved some level of muscle fatigue since MVC was conducted numerous times before obtaining results. This could be as to why our participant was generating just under the appropriate percentages of submaximal force. The corresponding EMG to the submaximal trials of mild, medium and strong were 33%, 49% and 85% respectively. The percentages from force compared to the corresponding percentages from EMG are similar. This allows us to interpret that there is a positive relationship between force and EMG. When there is an increase in force we will see a similar increase in EMG this is due to spatial and temporal summation. Spatial recruitment is the increasing recruitment of motor units and temporal recruitment refers to the rate code, in which active motor units are being fired at a greater frequency (L. Brent, personal communication, November, 2017). This summation grows simple twitches into tetanic or complete tetanus (L. Brent, personal communication, October 23rd, 2017). Thus, due to the muscle being in a steady state of contraction without relaxing there is a greater force being generated (L. Brent, personal communication, November, 2017).
A filter is used to process EMG signal in order to obtain signals produced only by EMG as surface electrodes may collect environmental noises or artifacts (De Luca et al., 2010). In the software Logger Pro a low-pass filter at any amount of hertz was dual passed. Dual pass means that instead of filtering through the data one time in one direction, known as a single pass, it is filtered a second time by passing through the data in the reverse direction (L. Brent, personal communication, November 27th, 2017). The result in a duel pass creates more filtering and more accuracy. It also shifts the data back to its original placement after being shifted on the time axis to the right in a single pass (L. Brent, personal communication, October 27th, 2017). This shift may be useful when comparing graphs of EMG and force. In a force graph there is a delay before it rapidly increases. This delay is due to the stimulus going to motor units first before it can release Ca2+ to contractile elements (L. Brent, personal communication, October 27th, 2017). A single pass then results in an alignment of the EMG signalling to when force is generated (L. Brent, personal communication, October 27th, 2017). A low-pass filter was used to cut out high frequency EMG. This smoothed out our data. When a low-pass filter at 10 Hz was used, shown in figure 6, it was noticeably more rigid compared to when a low-pass filter at 4 Hz was used, shown in figure 7. Therefore, we used the data from the low-pass filter at 4 Hz to graph against force (figure 8) this allowed for the most linearity. This is because low pass filtering at 10 Hz takes values more proximal values from one another and plots their average, when compared to low-pass filter at 4 Hz these taken values are more distal from one another (L. Brent, personal communication, October 27, 2017).
A limitation to normalizing our participant’s EMG to a percent maximum is that it will only be as good as the EMG’s MVC. Such that if the recorded MVC was not the maximal force the participant could generate it would result in a submaximal EMG recording that was denoted as maximal recording. Since the true maximal is unknown and normalizing other submaximal values to this ‘submaximal’ value would produce high values since the greatest activity that could be achieved was not recorded but still exists. Problems that may have occurred in the participant that could lead to a faulty MVC include, muscle fatigue, and participants posture and motivation. This in addition to the BB being a heterogenous muscle, could be as to why our participants force percentages were lower to the corresponding percentage of EMG MVC. Heterogenous muscles contains mixed muscle fibres and contribute more to the EMG as we get closer to MVC in compared to homogenous muscles (L. Brent, personal communication, October 25, 2017). This is because EMG signalling is best record in the superficial muscle and high velocity motor units are more concentrated superficially we record the activity of the last motor units to be recruited. This compensates for synergist muscles to BB and the number of motor units within BB being recording since these factors outcome produces a higher force compared to EMG and lower EMG amplitude recordings, respectively. Therefore, our participant achieved a higher than expected linearity relationship of force/EMG.
Our study confirmed there is a positive relationship between force and EMG. This correlation was not perfectly linear which was expected but was very close. This is indicated in Figure 8 with the line that best fits as it a great representation of the linearity of EMG/force in our participant. The relationship exists due to an increasing force requires more spatial and temporal recruitment. This summation of recruitment equals to higher EMG amplitude.
References
De Luca, C. J., Donald Gilmore, L., Kuznetsov, M., & Roy, S. H. (2010). Filtering the surface EMG signal: Movement artifact and baseline noise contamination. Journal of Biomechanics, 43(8), 1573–1579.
Enoka, R.M. (2015). Neuromechanics of human movement 5th edition. Champaign, IL, United States: Human Kinetics.
Gygi, A. E., & Moschytz, G. S. (2002). Low-pass filter effect in the measurement of surface EMG. Proceedings of Computer Based Medical Systems, 183–188.
Maton, B., & Bouisset, S. (1977). The distribution of activity among the muscles of a single group during isometric contraction. Europ. J. Appl. Physiol, 37, 101–109.
LAB 6: EMG/FORCE RELATIONSHIP
LESLIE CARSON
ID # 0930093
Date submitted: 07/11/2017
Lab group members: Emily Cianci, Derek Mak, Joseph Tabile
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more