The detection and characterization of QoI-resistant pathogens causing ascochyta blight of pulse crops in Montana
Abstract
Ascochyta blight (AB) of pulse crops (chickpea, field pea, and lentils) causes yield loss in Montana, where 1.2 million acres was planted to pulses in 2016. Pyraclostrobin and azoxystrobin, quinone outside inhibitor (QoI) fungicides, have been the choice of farmers for the management of ascochyta blight in pulses. However, a mutation in the cytochrome b gene has been reported to confer resistance to QoI fungicides. A total of 990 isolates of Ascochyta blight-causing fungi were isolated and screened for QoI resistance. Out of these, 10% from chickpea, 81% were from dry peas, and 9% from lentil. These were from a survey of growers fields and seed lots (chickpea = 17, pea = 131, and lentil = 21) from 23 counties in Montana sent to the Regional Pulse Crop Diagnostic Laboratory, Bozeman, MT for testing. Fungicide-resistant Didymella rabiei isolates were found in one chickpea seed lot each sent from Daniels, McCone and Valley Counties, MT, from seed produced in 2015 and 2016. Multiple alignment analysis of cDNA and protein sequences revealed a mutation that changed the codon for amino acid 143 from GGT to GCT, introducing an amino acid substitution from glycine to alanine (G143A), which is frequently associated with QoI resistance. Under greenhouse conditions, QoI-resistant isolates of D.rabieicaused significantly higher amounts of disease than sensitive isolates on pyraclostrobin-treated chickpea plants (p-value = 0.001). These results suggest that disease control may be inadequate at locations where resistant isolates are present. D. rabiei-specific polymerase chain reaction primer sets and TaqMan probes were developed to efficiently discriminate QoI-resistant and – sensitive isolates
Keywords: Ascochyta blight; pyraclostrobin; QoI-fungicide resistance; G143A mutation; hydrolysis probe assay
Introduction
The production of cool season pulse crops including chickpea (Cicer arietinum L.), pea (Pisiumsativum L.), and lentil (Lens culinaris Medik) in the Northern Great Plains of the United States is rapidly increasing. Montana is the leading producer of peas and lentil in the US, where 1.2 million acres were planted to pulses in 2016 (United States Department of Agriculture & National Agriculture Statistics Service 2016). However, an increase in pulse production is accompanied by potentially yield-limiting diseases. Chief among these diseases is Ascochyta blight (AB). This is a host-specific disease caused by fungal species including Didymella rabiei (Kovachevski) v. Arx (anamorph Ascochyta rabiei (Pass) Labr) on chickpea, a species complex consisting of Didymella pisi (Barilli et al., 2016), Peyronellaea pinodes, and Peyronellaea pinodella on dry pea,andDidymella lentis Kaiser, Wang and Rogers (anamorph A. lentis Vassiljevsky) on lentil (Barilli et al., 2016). AB can infect plants crop at all stages of development and cause over 40-50% yield reduction under conditions favorable for disease development (Wunsch, 2013; Wise et al., 2011; and Mondal et al., 2005). Yield losses caused by AB are in order of 40% in lentil (Barilli et al., 2016; and Gossen and Derksen, 2003), but in severe cases, losses higher than 90% have been reported in chickpea (Barilli et al., 2016; and Pande et al., 2005). Symptoms of AB can develop on all above-ground parts of the plant and also cause seed rot. AB is seed- and residue-borne. In the field, disease onset is normally post-flowering (growth stage R1) through plant maturity (growth stage R8). Infected seeds from diseased pods may be small, shrunken or discolored (Gossenet al., 2011; and Ye et al., 2000). In addition to seed as a source of inoculum, D. rabiei, D. pisi, and P.pinodes also can overwinter in the sexual and/or asexual forms (pseudothecia, pycnidia, and perithecia respectively), producing ascospores and conidia that can disperse and cause outbreaks of disease (Wise et al., 2011, Chilvers et al., 2008 and Tivoli and Banniza, 2007).
Management of AB requires an integrated approach including the use of certified disease-free seeds, sowing depth, crop rotation, burial of plant debris from previous planting season to prevent overwintering of the inoculum, fungicide seed treatment, use of resistant cultivars and foliar fungicides (Wise et al., 2011). The use of resistant varieties and cultural practices can reduce AB, however, resistant varieties are not widely available in the Northern Great Plains. Fungicides are widely used to achieve an acceptable level of disease control (Lonergan et al., 2015; and Davidson and Kimber, 2007). Broad spectrum protectant fungicides (chlorothalonil) is typically applied pre-flowering and can delay the onset of AB; however, once symptoms appear it is imperative for the grower to apply fungicides that provide a high level of control and move beyond the site of application in plant tissues due to canopy closure (Lonergan et al., 2015; Wise et al., 2009; Davidson and Kimber 2007; and Gan et al., 2006). This concern is heightened in chickpea which is more susceptible to AB when compared with dry peas and lentils. Thus, fungicides are frequently applied to chickpea fields and sparingly in peas and lentil fields. Three registered fungicide classes that provide a premium level of control for the management of AB include quinone outside inhibitors (QoI; Fungicide Resistance Action Committee [FRAC] code 11), demethylation inhibitors (DMI; FRAC code 3), and succinate dehydrogenase inhibitors (SDHI; FRAC code 7) (Lonergan et al., 2015; and Burrows, 2013). All of these fungicides are considered to have high to medium risk of resistance development (Fungicide Resistance Action Committee 2016). This concern is elevated by the site-specific mode of action (MOA) of the fungicides, the polycyclic nature of the disease, airborne spores of the AB pathogens, and of the option of sexual reproduction for most species, allowing rapid genetic evolution. The polycyclic nature of the disease predisposes growers to repeated fungicide application as disease severity can increase rapidly when the weather is favorable, particularly with AB of chickpeas (Fungicide Resistance Action Committee 2016; Lonergan et al., 2015; and Banniza et al., 2011).
Currently, of the three classes of fungicide, QoI fungicides are the choice of most pulse growers for pre- and post-infection management of AB in the United States and Canada (Lonergan et al., 2015; Bowness, 2016; Delgado et al., 2013; and Wise et al., 2008). Prior to 2007, it was the only available fungicide mode of action on pulse crops and resistance developed rapidly in North Dakota and Canada (Bowness, 2016; Wise, 2011; and Gossen, et al., 2004). In 2012, SDHIs were registered for use and these have largely been released as blends with another fungicide MOAs due to the high risk of resistance development.
In addition, grower’s preference of QoI- fungicides for disease control in pulse fields got a boost when the US Environmental Protection Agency (EPA) approved the use of pyraclostrobin (Headline®) to benefit plant health on federally issued labels (Agweb, 2009). This plant health benefit of QoI-fungicide was reported by Dimmock et al., (2002) to prolong grain filling in wheat crop. In addition, QoI-fungicide was reported to lower transpiration rate and also reduced rate of senescence in wheat plant (Mahoney, et al., 2014; and Petit, et al., 2012).
This fungicide inhibits mitochondrial respiration by binding to the center of the quinine (Qo) site of the cytochrome bc1 complex (complex III) on the positive side of the inner mitochondrial membrane. This causes depletion of adenosine triphosphate (ATP) that ultimately halts spore germination as a result of energy deficiency (Delgado et al., 2013; Wise et al., 2009; and Grasso et al., 2006a). Sequential to the registration of pyraclostrobin in 2003, it has become the most widely used fungicide for the control of AB in the US and Canada as both a seed treatment and foliar product (Bowness, 2013; and Wise et al., 2008).
Resistance to QoI fungicides has been reported in wheat pathogens such as Blumeriagraminis f. sp. tritici, Microdochium nivale, Microdochium majus, and Ourcosphaerella graminicola (Patel et al., 2012; Walker et al.,2009; Amand et al., 2003; and Sierotzki et al., 2000), and several other fungal pathogens including Cercospora sojinia, Botrytis cinerea, Alternaria alternata, Colletotrichum graminicola, Pyricularia grisea, Pythium aphanidermatum, Podosphaera fusca, Pyrenophora teres and Pseudoperonospora cubensis (Zeng et al., 2015; Samuel et al., 2011; Banno et al., 2009; Sierotzki et al., 2007; Avila-Adame et al., 2003; Ma et al., 2003; Kim et al., 2003; Gisi et al., 2002; and Ishii et al., 2001). In addition, QoI resistance has been reported in D. rabiei in North Dakota and Canada (Delgado et al., 2013; Wise et al., 2009; and Gossen et al., 2004). The mechanism of resistance of D. rabiei has been attributed to single amino acid substitutions in the cytochrome b protein of the cytochrome bc1 complex (Delgado et al., 2013). There are two types of fungicide insensitivity: quantitative and qualitative. Quantitative insensitivity results in the pathogen becoming less sensitive to the fungicide, although higher rates or more fungicide applications are still effective. Qualitative insensitivity causes the pathogen to become completely insensitive to the active ingredient and control is no longer possible at field rates. Insensitivity to the strobilurin fungicides is generally qualitative (Bowness et al., 2016). Presently, three amino acid substitutions are found in the cytochrome b protein of fungal plant pathogens that confer different levels of resistance to QoI fungicides (Delgado et al., 2013; and Grasso et al., 2006a). Low levels of resistance are conferred by a change from phenylalanine to leucine at position 129 (F129L) and a change from glycine to arginine at position 137 (G137R) while a high level of resistance is conferred by the amino acid substitution from glycine to alanine at position 143 (G143A). QoI resistant isolates have a transversion from GGT to GCT on their cytb that resulted in a mis-sense point mutation that substituted the amino acid from glycine to alanine at position 143 (G143A). QoI-resistant D. rabiei isolates have been identified in North Dakota and Montana (Wise et al., 2009; and Wise et al., 2008), where the mechanism of resistance was identified as the G143A mutation (Delgado et al., 2013). However, in Montana, there has not been a statewide survey to monitor for resistance to QoI in pulse crops. There is an urgent need to develop a robust screening and monitoring strategy for QoI resistance to help prevent the spread of QoI-resistant AB pathogens in the rapidly increasing pulse acreage in Montana. Thus, the objectives of this study were to 1) conduct a statewide survey to determine the incidence and severity of AB in pulse crops in Montana; 2) determine the frequency of resistance to QoI fungicides in AB pathogens from chickpea, pea, and lentil; 3) determine the mechanism of resistance associated with QoI-resistant isolates; and 4) develop a robust multiplex real-time PCR diagnostic tool for screening and monitoring of QoI resistance.
Materials and methods
A collection of D. rabiei, D. pisi, and D. lentis isolates.
Isolates of D. rabiei, D. pisi and D. lentis were obtained from four general sources. Most isolates were obtained from chickpea, field pea and lentil seed lots submitted by growers in 23 Montana counties to the Regional Pulse Crop Diagnostics Laboratory (RPCDL) in Bozeman, MT for planting during 2014, 2015, and 2016 growing season. A second set of isolates were obtained from chickpea and pea production fields in Montana where QoI fungicides had been applied. Fields containing chickpea and pea plants with ascochyta blight symptoms were sampled on a “W” pattern, with samples taken at the set of intervals of approximately 15 m. The third set of isolates were collected from chickpea and pea plants with ascochyta blight symptoms sampled by growers and submitted to the Schutter Diagnostic Laboratory, Bozeman, MT. Finally, some isolates were also obtained courtesy of Julie Pasche at North Dakota State University, Fargo, ND.
For a standard AB seed test, seed (chickpea n = 600, dry pea = 400 and lentil= 400) were sterilized in a 1% free chlorine solution for 10 minutes (ISTA, 2017). The solution was drained and sterilized seeds were air-dried in the biological cabinet for 30 minutes. Dried seeds (n=10 per plate) were plated on potato dextrose agar (PDA) (Alpha Biosciences Inc., Baltimore, MD). Mycelial growth was observed from the plated seeds after 11 to 14 days incubation at 20°C +/- 1oC under a diurnal cycle of cool white fluorescent light (12 h light followed by 12h dark). The presence of AB pathogenswas confirmed by microscopic observation of conidia at ×40 magnification.
From plants, isolates were also obtained from a single lesion on symptomatic leaves and stem by cutting the stems or leaves in 3- to 4-cm sections. Stem or leaf sections were placed in 1% NaOCl for 30 s and rinsed for 30 s in sterile distilled water. Sterilized stem or leaf sections were air-dried in a biological cabinet, placed on PDA and incubated under the conditions described above. Confirmation of the pathogen was conducted as previously described. Conidium of individual isolates from infected seed lots (n= 5 to 10) and from symptomatic leaves or stems were incubated on PDA under the conditions previously described. To isolate single spores, three pycnidia from 10 days old culture were dropped into 2ml screw cap tubes (MP Biomedicals) containing five ceramic beads (MP Biomedicals), 300 µl of sterile water and 0.05% (v/v) tween-20. The mixture was homogenized using a Beadbug homogenizer (Benchmark BeadBug Homogenizer) for 60 seconds at 4000 rpm. The supernatant was removed into a clean 1.5 ml eppendorf tube and diluted 100 fold in sterile water. From the diluted suspension, 100 µl was inoculated on fresh PDA plates and incubated at 20°C +/- 1oC under a diurnal cycle of cool white fluorescent light (12 h light followed by 12h dark). Single spores germinated after 3 – 5 days post-inoculation. Isolates were preserved for long-term storage as conidia on sterile filter paper and also as mycelia in 15% sterilized glycerol at –80°C. (Skoglund et al., 2011)
Screening of Ascochyta spp. isolates for QoI fungicide resistance using a discriminatory dose.
A total of 984 Ascochyta isolates were screened for QoI resistance. Of these, 10% were from chickpea, 81% from dry peas, and 9% from lentil from seed lots (chickpea = 17, pea = 131, and lentil = 21) submitted to the RPCDL from 23 counties in Montana (Table 1). Screening of the isolates was conducted using an in vitro agar plate method according to published methods (Wise at al., 2008) with some modifications. Stock solutions of technical grade formulations of pyraclostrobin (99% active; BASF Corporation, Research Triangle Park, NC) were prepared at a concentration of 5µg/ml and diluted in acetone. Salicylhydroxamic acid (SHAM; Sigma-Aldrich) was dissolved in methanol and added to all fungicide-amended media at a concentration 100 μg/ml to minimize the effects of the alternative oxidative pathway that some fungi use to overcome QoI fungicide toxicity in fungicide sensitivity assays in-vitro (Wise et al., 2009; Wise et al., 2008; and Bartlett et al., 2002; and Olaya et al., 1999). D. rabiei and other AB pathogens are able to use this alternative pathway in the presence of QoI fungicides, and SHAM has been determined to have no effect on conidial germination (Wise et al., 2008). The 0 μg/ml treatment served as a control and was amended with 100 μg/ml SHAM, 1 ml of acetone, and 1 ml of methanol per liter.
In addition to agar plate methods, isolates were screened using a mismatch amplification mutation assay PCR (MAMA-PCR) (Delgado et al., 2013). This PCR-based assay was developed to detect wild type isolates of D. rabiei bearing the G143 allele of the cytochrome b gene. Isolates that had a mycelial growth that is 70% of the control plate (without pyraclostrobin fungicide) and that also amplified with the MAMA-PCR were selected for total RNA extraction. Selected isolates were cultured on PDA at 22oC for 7 days at 12 h light.
Total RNA extraction
Total RNA was isolated using the RNeasy Plant Mini kit (QIAGEN) with modifications in the initial process. Fresh fungal mycelium from each isolate (100 mg) from a 7-day old culture was transferred to 2 mL screw cap tubes (MP Biomedicals) containing 450 µL RLC buffer. The mycelium was disrupted using the Beadbug benchtop homogenizer (Benchmark Scientific) set at speed 3500rpm for 60 s, and centrifuged at 13, 000 g for 1 min. About 400 µL lysate was then transferred to a QIAshredder spin column placed in a 1.5-mL collection tube. From this point onwards, the protocol continued according to the manufacturer’s instructions. Total RNA was quantified using a NanoDrop 2000c at 260 nm (Thermo Scientific) and brought to a final concentration of 100 ng/µL.
Synthesis of complementary DNA, RT-PCR, and sequencing
First-strand complementary DNA (cDNA) was synthesized using a RevertAid-Reverse Transcriptase kit (Thermo scientific). The cDNA was used in a PCR assay to amplify the coding sequence for amino acid codons 127–276 of the cytochrome b gene (cytb) from D. rabiei, this region has been reported to have point mutations that confer resistance to QoI fungicides (Delgado et al., 2013; and Fraaije et al., 2002).
Standard PCR was conducted in a T100 Biorad thermocycler (Bio-Rad Inc.) with Phusion High-Fidelity PCR master mix, 10 pmol each of primer (Delgado et al., 2013) and 50 ng of cDNA template in a final volume of 50 µL. The reaction conditions were: 94°C for 5 min, followed by 35 cycles at 94°C for 30 s, 55°C for 1 min and 72°C for 1 min. PCR was terminated with a DNA extension at 72°C for 5 min. PCR products were separated on ethidium bromide-stained 1.5% (w/v) agarose gels run in the 1x tris-acetate-EDTA buffer and exposed to UV light to visualize DNA fragments. Isolates with an expected product of 675 bp were purified directly from PCR products using alcohol precipitation. The purified PCR products were sequenced with primers used for the amplification (Table 2), in both directions (McLab DNA sequencing services).
Effect of G143A mutation on D. rabiei fungicide sensitivity on disease control on chickpea
Greenhouse trials were performed to determine the level of in-vivo disease control attainable with QoI fungicides against isolates classified as susceptible or resistant to QoI fungicides based on sequencing results. Five QoI-sensitive D. rabiei isolates (AR-405, AR-407, AR-419, AR-439, and AR-430) (Wise, 2008) and five QoI-resistant isolates (AR-R001 to AR-R005) were included in the trial (Table 5). The five QoI-resistant isolateswere isolated from one chickpea seed lot submitted to the RPCDL for seed test, the five QoI-sensitive isolates were from baseline population (Lonergan et al., 2015).TheQoI sensitivity of these five isolates was determined using pyraclostrobin amended PDA, MAMA PCR and mutation analysis of their cytb gene.
The greenhouse experiments were performed following Pasche et al. (2005, 2004) and Wise et al., 2009. Briefly, chickpea seeds (cv. Troy) received from Washington State Crop Improvement Association (WSCIA) and tested free of ascochyta blight were sown at one plant per pot in 80-ml plastic cones filled with a mixture of peat and Sunshine Mix 1 (Sun Gro Horticulture Inc., Bellevue, WA) at ratio 1:1, and grown at 22 ± 2°C. Ten to 14 days after planting, chickpea plants were treated with commercial formulations of pyraclostrobin (Headline, 2.09 EC; BASF Corporation) at concentrations of 0, 0.1, 1.0, 10, and 100 μg a.i. /ml of water. Fungicides were applied to runoff using a CO2-powered Generation III Research Track sprayer (DeVries Manufacturing, USA). Approximately 24 h after fungicides were applied, plants were inoculated with D. rabiei conidial suspensions prepared from 14-day-old cultures of selected sensitive and resistant isolates. Suspensions were adjusted to a concentration of 3 × 105 conidia/ml and applied to chickpea plants within an hour after preparation. Inoculum from each isolate was applied to plants using hand-held spray bottles. Chickpea plants were placed in a mist chamber and held at >90% relative humidity for 36 h at a 14-h photoperiod under artificial lighting before being placed on greenhouse benches. After 11 days, disease severity for plants was visually assessed as the percent leaf area infected of the whole plant (Reddy, 1984). The experiment was designed as a randomized complete block (RCB). Nine replicates were included in each experiment, and the disease severity was calculated for each observational unit. Percent disease control was calculated by: [1 – (% diseased tissue/ % disease on 0 μg/ml control)] × 100 (Wise et al., 2009). Homogeneity of the variances from the two greenhouse experiments was determined by Levene’s test of homogeneity of variance. Data were converted to percent disease control to facilitate direct comparisons between sensitive and resistant isolates at each fungicide concentration and analyzed using the generalized linear mixed-effects model in R statistical package.
Development of a multiplex hydrolysis probe assay for the detection of QoI-sensitive and QoI-resistant (G143A) D. rabiei isolates
For simultaneous detection and differentiation of the D. rabiei QoI-resistant isolates carrying the G143A mutation from the sensitive isolates, a primer pair (A243 and A244) and two hydrolysis probes (A245res and A246ses) were designed for a multiplex real-time PCR assay to amplify a 92bp fragment of the cytb gene (Table 2). The 5’ ends of the probes A245res and A246ses were labeled with 6-carboxy fluorescein (FAM) and cyanine 5 (Cy5), while their 3’ ends were labeled with Iowa black-FQ and Iowa black-RQ quencher, respectively. The primers were designed to flank the region of the G143A mutation, while the two fluorogenic dyes enable the multiplex differentiation between resistant and sensitive alleles. To enhance the efficiency of the probes both probes were designed to have Tmvalues at least 7oC higher (69 and 69oC) than that of the primers (62oC), and their GC content was higher than 45%. The multiplex TaqMan assay was optimized in a final volume of 20μL containing 0.05 U/μL Taq DNA polymerase, reaction buffer, 4 mM MgCl2, 0.4 mM of each dNTP (Invitrogen Life Technologies, CA), 25pM of each primer (A243, A-244), 10pM of each probe (A-245res, A-246sens) and 3μL of DNA extract. Cycling parameters were 4min at 94oC, followed by 35 cycles of 15s at 94oC and 30s at 64oC and a final extension at 72oC for 5minutes completed the PCR. Primers and TaqMan probes were synthesized by Integrated DNA Technology (Iowa, USA). The assay was developed, evaluated and analyzed on the Biorad CFX96 real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA).
The capability of the developed assay was further investigated by screening isolates of D. pisi, D. lentis and other fungal species that have been reported to contain the G143A mutation, such as Alternaria alternata. For this experiment, 100 ng of DNA from each of the species was used, isolates of each species were replicated once in the assay. QoI-resistant and sensitive D. rabiei isolates were used as positive controls, and the cycling parameters stated above was used in the assay. In addition, evaluation of the assay efficiency was determined by plotting cycle thresholds for a tenfold dilution series of 1000 ng DNA obtained from sensitive and resistant isolates against DNA concentration to yield the standard curves. The result from experiments in the multiplex assay was compared to the uniplex assay using tenfold dilutions from 1000 ng to 1 pg DNA extracts from only isolates that contained the G143A mutation.

Figure 1. The partial protein sequence of the cytochrome b gene of five isolates of Didymella rabiei with different QoI sensitivities. Star indicates the G143A amino acid substitution responsible for decreased sensitivity to QoI fungicides. Dark gray highlighted areas represent 100% identities.
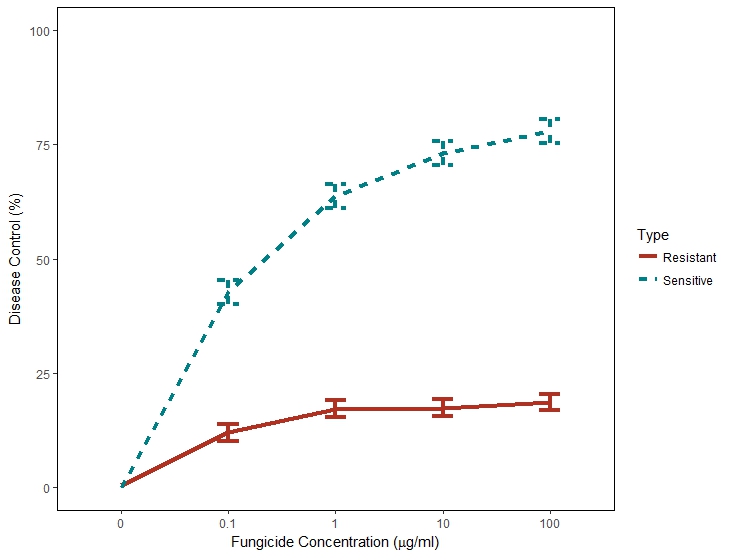
 Figure 2. Mean in-vivo percent disease control for five QoI-sensitive (—–) and five QoI-resistant ( ) Didymella rabiei isolates to pyraclostrobin fungicide concentration (μg/ml). Values include standard errors of disease control measurements obtained from one plant across nine replications in two experiments.
Figure 2. Mean in-vivo percent disease control for five QoI-sensitive (—–) and five QoI-resistant ( ) Didymella rabiei isolates to pyraclostrobin fungicide concentration (μg/ml). Values include standard errors of disease control measurements obtained from one plant across nine replications in two experiments.
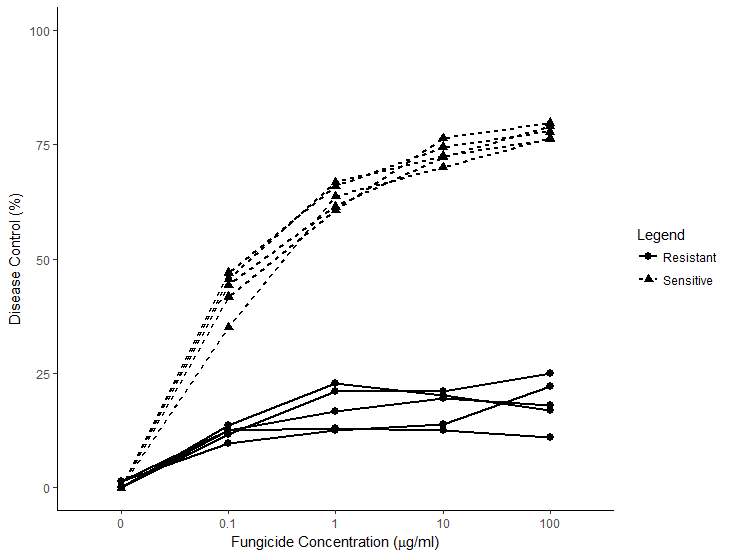
Figure 3. Mean in-vivo percent disease control for five QoI-sensitive and five QoI-resistant Didymella rabiei isolates to pyraclostrobin fungicide at all concentration (μg/ml).
 FAM Resistant probe
FAM Resistant probe
 Cy5 Sensitive probe
Cy5 Sensitive probe
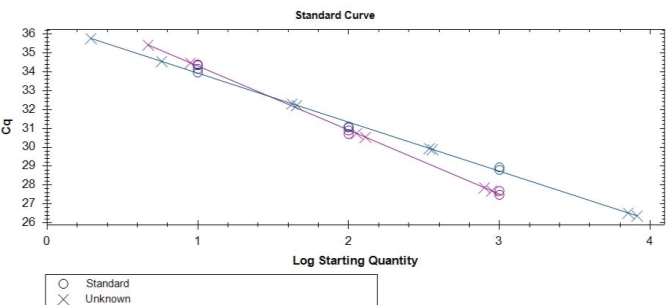
Figure 4. Standard curve obtained by using the multiplex SNP TaqMan assay to detect the G143A mutation in D. rabiei isolates collected from Montana.
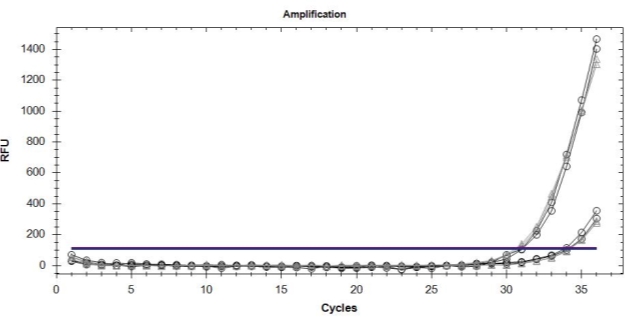
A
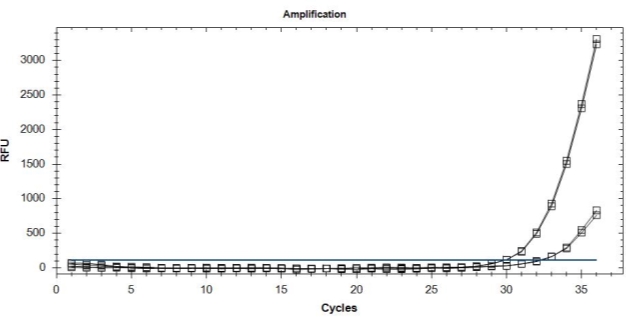
B

 Figure 5. (A) Amplification curves showing the detection efficiency of sensitive (o) and resistant (––) alleles using the mixtures of DNA from QoI-resistant and QoI-sensitive isolates as a template. (B) Amplification curves showing the detection efficiency of resistant ( ) alleles in uniplex TaqMan real-time PCR using DNA from QoI-resistant isolates as a template.
Figure 5. (A) Amplification curves showing the detection efficiency of sensitive (o) and resistant (––) alleles using the mixtures of DNA from QoI-resistant and QoI-sensitive isolates as a template. (B) Amplification curves showing the detection efficiency of resistant ( ) alleles in uniplex TaqMan real-time PCR using DNA from QoI-resistant isolates as a template.
Results
Multiple alignment analysis of cDNA and protein sequences of the cytochrome b gene of the QoI-resistant isolates revealed a mutation that changed the codon for amino acid 143 from GGT to GCT, introducing an amino acid substitution from glycine to alanine (G143A) (Figure 1). Other known mutations were not found in the protein sequences of our QoI-resistant isolates, mutations such as a change from phenylalanine to leucine at position 129 (F129L) and a change from glycine to arginine at position 137 (G137R).
Effect of G143A mutation on D. rabiei fungicide sensitivity
Independent analysis of greenhouse disease control experiments determined that variances were homogeneous and the two experiments were combined for further analysis (p-value =). Disease severity was significantly greater on plants inoculated with G143A mutant isolates at all concentrations of pyraclostrobin, including the non-treated control (0 μg/ml); therefore, percent disease control from the non-treated was calculated to directly compare the two isolate groups. There was convincing evidence of a difference in the percent disease control in the interaction of G143A mutants and wild type D. rabiei isolates and pyraclostrobin fungicide (p-value < 0.001). There was also evidence of a difference in the percent disease control in chickpea plants inoculated with G143A mutants and wild type D. rabiei isolates with increasing concentration of pyraclostrobin fungicide (p-value< 0.001).
Disease control of G143A mutant isolates was significantly reduced in the pyraclostrobin treatments when compared to wild type isolates at all fungicide concentrations (p-value < 0.001). (Figure 3). About 75% disease control was observed at 10 and 100µg/ml in wild-type isolates while < 25% disease control was observed in G143A mutant isolates.
Detection of QoI-sensitive and QoI-resistant (G143A) D. rabiei isolates using a multiplex hydrolysis probe assay
By using the SNP TaqMan assay, it was possible to detect the G143A mutation and discriminate between QoI- resistant and QoI- sensitive isolates. The SNP could be distinguished using specific probes in which a nucleotide proximal to the 3’ is complementary to one allele but forms as a mismatch with the second allele. The annealing temperature was validated in a temperature gradient assay, the optimum annealing temperature is 64oC for both multiplex and uniplex (data not shown). The optimum concentration of primers and probes that gave the highest reporter fluorescence and the lowest threshold cycle was 20 and 10 µM, respectively, in both tests. To confirm the assay can simultaneously detect the two alleles, DNA from wild-type (QoI-sensitive) and mutant (QoI-resistant) isolates were mixed in the same proportion. Satisfactory discrimination was achieved between the two alleles (Figure 5A and 5B). Standard curves were constructed based on the tenfold dilution series of the wild-type and mutant isolates (Figure 4). The linearity of the amplification across the dilutions was confirmed, and the correlation coefficients for the standard curves of DNAs from sensitive and resistant isolates were 0.998 and 0.991, respectively (Table 4).
The assay was also validated using other fungi from the same genus as D. rabiei, such as D. lentis and D. pisi and other fungi reported to have G143A mutation, including Alternaria alternata. For all sensitive fungal species tested, there was only amplification in the sensitive probe. In addition, the robustness of the assay was validated using DNAs crudely extracted from D. rabiei, D. pisi, D. lentis, and A. alternata only the control sample had amplification from the two probes and the other fungi species only had amplification from the sensitive probe, showing that the isolates were QoI-sensitive and the primer/probe combination worked in these fungal species (Table 6).
Discussion.
Based on our results, the G143A point mutation is responsible for QoI fungicide resistance in D. rabiei isolates from Montana chickpea fields. The gene structure of the cytochrome b gene of D. rabiei appears to be favorable for the development of a point mutation related to QoI resistance at codon 143 (Delgado et al., 2013). In contrast, the G143A mutation has not been found in fungal species that have an intron downstream of codon 143 (Zeng et al., 2015; Delgado et al., 2013; Samuel et al., 2011; Banno et al., 2009; Sierotzki et al., 2007; and Grasso et al., 2006b). Thus, this cytochrome b gene structure has not been associated with the occurrence of QoI resistance. For example, fungal species such as Alternaria solani do not exhibit a G143A mutation because the 5’-splice site is lethally affected by the nearby exonic flanking sequences (Delgado et al., 2013; Banno et al., 2009; Sierotzki et al., 2007; Grasso et al., 2006b; Pasche et al., 2005; Lambowitz and Belfort, 1993). However, G143A mutation has been reported to be responsible for QoI fungicide resistance in Cercospora sojina, the causal organism of frog eye leaf spot of soybean (Zeng et al., 2015) and in Botrytis cinerea, the causal organism of gray mold (Samuel et al., 2011). QoI fungicides do not control fungi bearing the G143A mutation, while those containing the F129L and G137R are controlled to some degree, but at a lower level than wildtype isolates. Our results are similar to a study in North Dakota (Delgado et al., 2013, and Wise et al., 2009) where the mechanism of resistance of QoI-resistant D. rabiei isolates was associated with a G143A mutation in the cytochrome b gene.
Resistance to QoI fungicides was observed in isolates of D. rabiei collected in Montana during the 2012 and 2015 growing seasons (Burrows, data not shown). Prior to this, QoI-resistant isolates were reported in 2009 from chickpea fields in North Dakota (Wise et al., 2009) and have been largely maintained in the population (Delgado et al., 2013). From both seed testing results and the current in-field survey of Montana pulse fields, the frequency of resistant isolates is thus far low (B. Agindotan, personal communication, March 2016). This is different from the report of high frequency of QoI-resistant isolates in North Dakota (Delgado et al., 2013; Wise et al., 2009). This may be due to the comparatively low relative humidity and disease progression in Montana vs. North Dakota. The relative humidity level in North Dakota is 12.1% higher than in Montana (ClimaTemps, 2015). Though this study was targeted at AB of chickpea, field pea, and lentil, only 11 isolates of D. rabiei from three seed lots out of 88 isolates from 17 seed lots, were confirmed to be resistant to pyraclostrobin, and contain the G143A mutation which confers resistance to all QoI fungicides. The frequency of QoI resistance might be on the rise in D. rabiei because, from observations during the 2016 crop year and grower testimonies, there is potential for increasing chickpea acreage and the multiple applications of fungicides to ward off potential fungal attacks. Many of these applications were QoI products solely and in combination with either chlorothalonil or SDHI fungicides such as (fluxapyroxad). Continuation of the practice of applying multiple application of fungicides including high-risk products such as QoI and SDHI fungicides will select for resistance development. These active ingredients are available as seed treatments and foliar products. Although education is underway, fungicide decisions are often driven by the price and efficacy of the product more than the mode of action.
In contrast with chickpea, dry pea and lentil fields are rarely treated with fungicides. This is due to the low foliar disease occurrence in Montana to date. Since seed testing was started in Montana in 2000, the percent of seed lots with at least one seed of 500 infected by AB has increased from 0% (2000-2002) to <25% through 2009. Since the 2010 crop year, the number of seed lots with at least trace levels of AB in seed has increased above 60% and in 2017, reached 80%. To date, we have not observed QoI resistance in AB pathogens recovered from a dry pea and lentil seed lot. This correlates with the low frequency of fungicide application in pea. Similarly, lentils often do not receive any fungicide seed treatments or foliar applications, particularly in newly planted acreage.
Differences in disease control were observed when pyraclostrobin was applied to chickpea plants infected with QoI-resistant and QoI-sensitive D. rabiei isolates. Applications of pyraclostrobin at a concentration of 100 μg/ml provided less than 25% control of disease on plants infected with QoI-resistant isolates. This level of control is commercially unacceptable. Clear differences in disease severity were observed between the QoI-sensitive isolates causing less disease on fungicide-treated chickpea plants as compared to the QoI-resistant isolates used in the study. It is well established that resistance to QoIs in field isolates of several plant pathogens, associated with the G143A substitution in cytb, do not entail a fitness cost (Veloukas et al., 2014; Karaoglanidis et al., 2011; Avila-Adame et al., 2003; and Chin et al., 2001). However, once established, resistance is likely to be preserved in the population due to the selective advantage if QoI fungicides continued to be applied. This lack of disease control in QoI-resistant isolates confirmed a report from North Dakota (Wise et al., 2009), where <50% disease control was achieved with the applications of 100 µg a.i/ml pyraclostrobin to chickpea plants infected with QoI-resistant isolates. In light of this, monitoring of QoI resistance in AB pathogens infecting pulse crops is important to prevent the establishment of resistant populations. Due to the widespread occurrence of AB pathogens in seed lots in Montana, favorable environmental conditions will likely lead to a widespread epidemic of the pathogen. Frequent fungicide applications in those circumstances will lead to the establishment of resistant isolates and then additional management strategies will be needed to be deployed to manage fungicide resistant AB pathogens. Although limited studies abound on the prevention and management of fungicide resistance. Various disease management strategies can significantly modify the inherent risk of fungicide resistance in the target pathogen. The risk can be lowered by limiting the number of applications of single-site fungicides with the same mode of action, rotation among fungicides with different biochemical modes of action or by using synergistic fungicide combinations. This approach was very effective in the control of fungicide-resistant strains of sclerotinia dollar spot and pythium blight in turfgrass disease (Couch, 2003 and 2002).
The development of QoI resistant isolates in the epicenter of pulse production in Montana could cause significant problems for the industry, which in 2016 occupied 1.2 million acres and was valued at $176 million dollars. To contain the spread of QoI- resistance, monitoring is key. PCR-based tools like the one developed in this study are needed to monitor the shift in fungicide sensitivity of field population of fungal pathogens. Furthermore, non-chemical management practices such as crop rotation, tillage to bury infested residues and host resistance (if available) can help reduce the risk of fungicide resistance (Zeng et al., 2015). Moreover, there should be caution in the application of foliar fungicides for reasons other than disease control, limit the number of QoI fungicide applications to two to four per season as specified on the label. Make preventative applications to keep disease pressure low. Limit the number of sequential applications of strobilurin fungicide to one or two, as specified on the label, before alternating with a fungicide from a different mode of action group, use pre-mixtures or tank mixtures of strobilurin fungicides with fungicides from a different mode of action group. The minimum labeled rates of each fungicide in the tank mix should be used (Damicone, 2009). Failure to practice the above-enumerated guidelines will apply additional selection pressure on D. rabiei and other potential fungal pathogens which will consequentially warrant continued monitoring of fungal pathogens for resistance to high-risk fungicides.
Several molecular methods have been developed and used for the detection of QoI resistance on the basis of the presence of the G143A mutation. The aim of most of these methods is a qualitative identification of the polymorphism, and they include allele-specific PCR, PCR-RFLP, and CAPS tests while quantitative methods such as allele-specific real-time PCR has been developed for a limited number of pathogens (Zeng et al., 2015 and Samuel et al., 2011). The real-time PCR assay enhances high throughput during QoI-sensitivity screening of isolates when compared to the conventional in vitro test using fungicide amended PDA plates. This test takes about 7 to 10 days to get a result, in addition to the logistics required to screen multiple isolates. In the present study, a real-time PCR system based on TaqMan chemistry for the multiplex detection of QoI-sensitive and QoI-resistant isolates of D. rabiei, D. pisi and D. lentis was developed and successfully applied to isolates collected from different geographical locations around Montana. The hydrolysis probes were designed to have Tmvalues at least 7◦C higher (69 and 69oC) than that of the primers (62oC), and their GC content was higher than 45%, enhancing the specificity of the assay in detecting and differentiating sensitive from resistant alleles. The assay has the potential to monitor QoI-resistance in A.alternata, thus it can serve as a tool to monitor QoI resistance in other fungal pathogens and also for routine screening of fungal isolates in diagnostic laboratories. In addition, this technique is suitable for future studies aiming to determine changes in the frequency of G143 and A143 alleles and also to determine the competitive ability of QoI-resistant and QoI-sensitive isolates of fungal pathogens.
In conclusion, this study was successful in detecting the presence of QoI- resistant D. rabiei isolates, characterize the mechanism of resistance and develop a diagnostic tool for QoI resistance in D. rabiei that will allow high throughput and efficient screening of G143A mutants. Other researchers have been successful in developing a molecular technique to detect G143A mutation (Delgado, 2013). This is a qualitative assay that cannot be used to monitor the frequency of G143 and A143 alleles both in individual isolates and in mixed field populations. The assays reported in this study streamlines the whole detection process by reducing the need for pre- and post-amplification manipulations, such as gel electrophoresis and the use of ethidium bromide or other toxic compounds, and thus the staff input required. This process could be related to large-scale surveys, as well as to the high-throughput identification of resistant isolates to QoI fungicides. Furthermore, this technique can be used in future studies aiming to determine changes in the frequency of G143 and A143 alleles both in individual isolates and in mixed field populations of D. rabiei and the competitive ability of QoI-resistant and QoI-sensitive isolates of the pathogen.
References
Agweb (2009). Headline® fungicide from BASF receives EPA approval for Plant Health label. Online publication. http://www.agweb.com/article/headline® fungicide from basf receives EPA approval for plant health label 200062/
Amand, O., Calay, F., Coquillart, L., Legat, T., Bodson, B., Moreau, J.-M., and Maraite, H. (2003). First detection of resistance to QoI fungicides in Mycosphaerella graminicola on winter wheat in Belgium. Commun. Agric. Appl. Biol. Sci. 68, 519-531
Aveskam, M. M., Gruyter, J. de., Woudenberg, J. H. C., Verkley, G. J. M., and Crous, P. W. (2010). Highlights of the Didymellaceae: A polyphasic approach to characterize Phoma and related pleosporalean genera Stud. Mycol. 65: 33 (2010)
Avila-Adame, C., Olaya, G., and Koller, W. (2003). Characterization of Colletotrichum graminicola isolates resistant to strobilurin related QoI fungicides. Plant Dis. 87:1426-1432
Avila-Adame, C., and Köller, W. (2003). Characterization of spontaneous mutants of Magnaporthe grisea expressing stable resistance to the QoI inhibiting fungicide azoxystrobin. Curr. Genet. 42:332-338
Banniza, S., Armstrong-Cho, C. L., Gan, Y. and Chongo, G. (2011) Evaluation of fungicide efficacy and application frequency for the control of ascochyta blight in chickpea. Canadian Journal of Plant Pathology, 33:2, 135-149,
Banno, S., Ymashita, K., Fukumori, F., Okada, K., Uekusa, H., Takagaki, M., Kimura, M., Fujimura, M., (2009). Characterization of QoI resistance in Botrytis cinerea and identification of two types of mitochondrial cytochrome b gene. Plant Pathol. 58, 120-129.
Barilli, E; Cobos, M. J; and Rubiales, D. (2016). Clarification on Host Range of Didymella pinodes the Causal Agent of Pea Ascochyta Blight. Front. Plant Sci. 7:592.
Bowness, R., Gossen, B. D., Chang, K.F., Goswani, R., Willenborg, C. J., Holtz, M., and Strelkov, S. E. (2016). Sensitivity of Mycosphaerella pinodes to pyraclostrobin fungicide. Plant Dis. 100:192-199.
Burrows, M. (2013). High Plains Integrated Pest Management: Pulse crop foliar fungicides. Online publication. http://wiki.bugwood.org/HPIPM
Chen, Q., Jiang, J.R., Zhang, G.Z., Cai, L., Crous, P.W. (2015). Resolving the Phoma enigma. Studies in Mycology. 82:137-217
Chilvers, M. I., Rogers, J. D., Dugan, F. M., et al. (2009). Didymella pisi sp. nov., the teleomorph of Ascochyta pisi. Mycological Research 113: 391–400
Chin, K. M., Chavaillaz, D., Kaesbohrer, M., Staub, T., and Felsenstein, F. G. (2001). Characterizing resistance risk of Erysiphe graminis f. sp. Tritici to strobilurins. Crop Prot. 20:87-96.
ClimaTemps (2015). http://www.fargo.climatemps.com/vs/great-falls.php
Couch, H. B. (2003). Golf Course Management. May. 71(5): p. 111-115
Couch, H. B. (2002). Golf Course Management. November. 70(11): p. 89-93.
Damicone, J., and Smith, D. (2009). Fungicide Resistance Management. Oklahoma Cooperative Extension Service. EPP-7663
Davidson, J. A., and Kimber, R. B. E. (2007). Integrated disease management of Ascochyta blight in pulse crops. Eur. J. Plant Pathol. 119:99-110.
Delgado J. A., Lynnes T. C., Meinhardt S. W., Wise K. A., Gudmestad N. C., Bradley C. A., Markell S. G., and Goswami R. S. 2013. Identification of the mutation responsible for resistance to QoI fungicides and its detection in Ascochyta rabiei (teleomorph Didymella rabiei). Plant Pathology 62, 688–697
Evaluation of the incidence of the G143A mutation and cytb intron presence in the cytochrome bc-1 gene conferring QoI resistance in Botrytis cinerea populations from several hosts. Pest Manag. Sci 2011; 67: 1029–1036
Fraaije, B. A., Butters, J. A., Coelho, J. M., Jones, D. R., and Holloman, D. W. (2002). Following the dynamics of strobilurin resistance in Blumeria graminis f.sp. tritici using quantitative allele-specific real-time PCR measurements with the fluorescent dye SYBR green I. Plant Pathol. 51:45-54.
Fungicide Resistance Action Committee. (2015). Fungicides sorted by mode of action. FRAC Code List. Online publication: http://www.frac.info/docs/defaultsource/ publications/frac-code-list/frac-code-list-2015-finalC2AD7AA36764. pdf?sfvrsn=4
Fungicide Resistance Action Committee. (2016). Fungicides sorted by mode of action. FRAC Code List. Online publication: www.frac.info/docs/…/frac…/frac-code-list-2016.pdf
Gan, Y. T., Siddique, K. H. M., Macleod, W. J., and Jayakumar, P. (2006). Management options for minimizing the damage by Ascochyta blight (Ascochyta rabiei) in chickpea (Cicer arietinum L.). Field Crops Res. 97:121-134.
Gisi, U., Sierotzke, H., Cook, A., and McCaffery, A. (2002). Mechanisms influencing the evolution of resistance to Qo inhibitor fungicides. Pest Manage. Sci. 58:859-867
Gossen, B. D., and Derksen, D. A. (2003). Impact of tillage and crop rotation on Aschochyta blight (Ascochyta lentis) of lentil. Can. J. Plant Sci. 83,411–415.doi: 10.4141/P02-088
Gossen, B. D., Anderson, K. L., (2004). First report of resistance to strobilurin fungicides in Didymella rabiei. Canadian Journal of Plant Pathology 26, 411
Gossen, B. D., Hwang, S. F., Conner, R. L., and Chang, K. F.(2011). Managing the ascochyta blight complex on field pea in western Canada Prairie. Soils & Crops Journal Volume 4 ▪ 2011
Grasso, B., Sierotzki, H., Garibaldi, A., and Gisi, U., (2006a). Characterization of the cytochrome b gene fragment of Puccinia species responsible for the binding site of QoI fungicides. Pesticide Biochemistry and Physiology 84, 72–82
International Rules for Seed Testing (2017). Validated Seed Health Testing Methods. 7‑005: Detection of Ascochyta pisi in Pisum sativum (pea) seed. https://www.seedtest.org/upload/cms/user/2017-SH-7-005.pdf
Ishii, H., Fraaije, B. A., Sugiyama, T., Noguchi, K., Nishimura, K., Takeda, T., Amano, T., and Hollomon, D. W. (2001). Occurrence and molecular characterization of strobilurin resistance in cucumber powdery mildew and downy mildew. Phytopathology 91:1166-1171.
Karaoglanidis, G. S., Luo, Y., and Michailides, T. J. (2011). Competitive ability and fitness of Alternaria alternata isolate resistant to QoI fungicides. Plant Dis. 95:178-182.
Kim, Y. S., Dixon, E. W., Vincelli, P., and Farman, M. L, (2003). Field resistance to strobilurin (QoI) fungicides in Pyricularia grisea caused by mutations in the mitochondrial cytochrome b gene. Phytopathology 93, 891–900
Lambowitz, A. L., and Belfort, M. (1993). Introns as mobile genetic elements. Annual Review of Biochemistry 62, 587–622.
Lonergan, E., Pasche, J., Skoglund, L., and Burrows, M. (2015). Sensitivity of Ascochyta species infecting pea, lentil, and chickpea to boscalid, fluxapyroxad, and prothioconazole. Plant Dis. 99:1254-1260.
Ma, Z., Felts, D., and Michailides, T. J. (2003). Resistance to azoxystrobin in Alternaria isolates from pistachio in California. Pestic. Biochem. Physiol. 77:66-74
Mahoney, K. J., Vyn, R. J., and Gillard, C. L. (2014). The effect of pyraclostrobin on soybean plant health, yield, and profitability in Ontario. Can. J. Plant Sci. (2014) 94: 1385-1389
Mondal, S. N., Bhatia, A., Shilts, T., and Timmer, L. W. (2005). Baseline sensitivities of fungal pathogens of fruit and foliage of citrus to azoxystrobin, pyraclostrobin, and fenbuconazole. Plant Disease. 89:1186-1194
Nass.usda.gov/Publications/Todays_Reports/reports/crop1116.pdf
Olaya, G., and Köller, W. (1999). Diversity of kresoxim-methyl sensitivities in baseline populations of Venturia inaequalis. Pestic. Sci. 55:1083-1088.
Pande, S., Siddique, K. H. M., Kishore, G. K., Bayaa, B., Gaur, P. M., Gowda, C. L. L., et al. (2005). Ascochyta blight of chickpea (Cicer arietinum L.): a review of biology, pathogenicity, and disease management. Aust. J. Agr. Res. 56,317–332. doi: 10.1071/AR04143
Pasche, J. S., Piche, L. M., and Gudmestad, N. C. (2005). Effect of the F129L mutation in Alternaria solani on fungicides affecting mitochondrial respiration. Plant Disease. 89:269-278
Patel, J. S., Gudmestad, N. C., Meinhardt, S., and Adhikari, T. B. (2012). Pyraclostrobin sensitivity of baseline and fungicide exposed isolates of Pyrenophora tritici-repentis. Crop Protection 34 (2012) 37-41
Petit, A. N., Fontaine, F., Vatsa, P., Clement, C. and Vaillant- Gaveau, N. (2012). Fungicide impacts on photosynthesis in crop plants. Photosynth. Res. 111: 315-326.
Samuel, S., Papayiannis, L. C., Leroch, M., Veloukas, T., Hahnc, M., and Karaoglanidis, G. S. (2011) Evaluation of the incidence of the G143A mutation and cytb intron presence in the cytochrome bc-1 gene conferring QoI resistance in Botrytis cinerea populations from several hosts. Pest Manag Sci 2011; 67: 1029–1036
Sierotzki, H., Frey, R., Wullschleger, J., Palermo, S., Karlin, S., Godwin, J., and Gisi, U. (2007). Cytochrome b gene sequence and structure of Pyrenophora teres and P. triticirepentis and implications for QoI resistance. Pest. Manage. Sci. 63:225-233.
Sierotzki, H., Parisi, S., Steinfeld, U., Tenzer, I., Poirey, S., and Gisi, U. (2000). Mode of resistance to respiration inhibitors at the cytochrome bc1 enzyme complex of Mycosphaerella fijiensisfield isolates. Pest Management Science 56, 833–41.
Skoglund, L. G., Harveson, R. M., Chen, W., Dugan, F., Schwartz, H. F., Markell, S. G., Porter, L., Burrows, M. L., and Goswami, R. (2011). Ascochyta blight of peas. Online. Plant Health Progress doi: 10.1094/PHP-2011-0330-01-RS.
Tivoli, B.,and Banniza, S. (2007).Comparison of the epidemiology of Ascochyta blights on grain legumes. Eur.J.Plant Pathol. 119,59–76.doi:10.1007/s10658- 007-9117-9
Veloukas, T., Kalogeropoulou, P., Markoglou, A. N., and Karaoglanidis, G. S. (2014). Fitness and competitive ability of Botrytis cinerea field isolate with dual resistance to SDHI and QoI fungicides, associated with several sdhB and the cytb G143A mutations. Phytopathology 104:347-356.
Walker, A. S., Auclair, C., Gredt, M., Leroux, P., (2009). First occurrence of resistance to strobilurin fungicides in Microdochium nivale and Microdochium majus from French naturally infected wheat grains. Pest Manag. Sci. 65, 906-915.
Wise, K. A., Bradley, C A., Markell, S., Pasche, J., Delgado, J. A., Goswami, R. S., and Gudmestad, N C. (2011). Sensitivity of Ascochyta rabiei populations to prothioconazole and thiabendazole. Crop Protection 30 (2011) 1000-1005
Wise, K. A., Bradley, C. A., Pasche, J. S., and Gudmestad, N. C. (2009). Resistance to QoI fungicides in Ascochyta rabiei from chickpea in the Northern Great. Plant Dis. 93:528-536
Wise, K. A., Bradley, C. A., Pasche, J. S., Gudmestad, N. C., Dugan, F. M., and Chen, W. (2008).
Baseline sensitivity of Ascochyta rabiei to azoxystrobin, pyraclostrobin, and boscalid. Plant Dis. 92:295-300.
Ye, G., McNeil D.L. and Hill, G.D 2000. Lentil Ascochyta blight and breeding for its resistance New Zealand Plant Protection 53:97-102 (2000)
Zeng, F., Amao, E., Zhang, G., Olaya, G., Wullschleger, J., Sierotzki, H., Ming, R., Blihm, B. H., Bond, J. P., Fakhoury, A. M., and Bradley, C. A (2015). Characterization of quinone outside inhibitor fungicide resistance in Cercospora sojina and development of diagnostic tools for its identification. Plant Dis. 99:544-550
Table 1. Isolates of D. rabiei, D. pisi, and D. lentis were obtained from chickpea, field pea and lentil seed lots sent by growers to the Regional Pulse Crop Diagnostics Laboratory (RPCDL) in Bozeman, MT for planting during 2014, 2015, and 2016 growing seasons
| Collection location by County | Number of seed lots sampled | Total number of isolates | Isolates with QoI resistance a |
| Cascade | 6 | 39 | 0 |
| Chouteau | 2 | 3 | 0 |
| Daniels | 28 | 235 | 5 |
| Dawson | 7 | 38 | 0 |
| Gallatin | 5 | 16 | 0 |
| Glacier | 2 | 5 | 0 |
| Hill | 7 | 45 | 0 |
| Liberty | 2 | 6 | 0 |
| McCone | 23 | 92 | 4 |
| Musselshell | 1 | 1 | 0 |
| Philips | 4 | 9 | 0 |
| Pondera | 5 | 6 | 0 |
| Richland | 3 | 16 | 0 |
| Roosevelt | 23 | 133 | 0 |
| Sheridan | 15 | 81 | 0 |
| Teton | 2 | 4 | 0 |
| Valley | 24 | 213 | 2 |
| Yellowstone | 2 | 9 | 0 |
| Toole | 1 | 2 | 0 |
| Broadwater | 1 | 2 | 0 |
| Flathead | 1 | 5 | 0 |
| Blaine | 2 | 15 | 0 |
| Garfield | 2 | 15 | 0 |
| Total | 168 | 990 | 11 |
a An isolate was considered resistant if it has the G143A mutation in its cytochrome b gene
Table 2. Primers used for amplification of the cytochrome b gene fragment of Didymella rabiei and for screening of the G143A mutation
| Primers | Primer sequence (5’-3’) | Annealing temperature | Reference | Primer pair purpose |
| A99 | TATTATGAGAGATGTAAATAATGG | 46oC | Delgado, 2013 | Sequencing of cytochrome b gene |
| A100 | CCTAATAATTTATTAGGTATAGATCTTA | 46oC | Delgado, 2013 | Sequencing of cytochrome b gene |
| A243 | GCTTTCCTGGGTTACGTTCT | 64oC | This study | Multiplex TaqMan PCR |
| A244 | CCAACTCATGGTATAGCACTCAT | 64oC | This study | Multiplex TaqMan PCR |
| A245res | FAM-TGGGCAAATGTCACTATGAGCTGCTACAG-BHQ1 | 64oC | This study | QoI-resistant probe (A143 allele) |
| A246sens | Cy5-TGGGCAAATGTCACTATGAGGTGCTACAG-BBQ | 64oC | This study | QoI-sensitive probe (G143 allele) |
Table 3. Distribution of Ascochyta isolates collected per crop in 2014-2016 from Montana
| Crop | Number of Counties sampled | Total Number of seed lots | Total number of isolates | Isolates with QoI resistance |
| Chickpea | 9 | 17 | 88 | 11 |
| Field pea | 17 | 130 | 810 | 0 |
| Lentil | 7 | 21 | 92 | 0 |
| Total | 168 | 990 | 5 |
Table 4. Slope, efficiencies, correlation coefficients (R2), and y-intercepts from amplification of serial dilutions of DNA from Didymella rabiei isolates sensitive and resistant to quinone outside inhibitor fungicides using TaqMan single-nucleotide polymorphism assay to detect G143A mutation
| Allele | Slope | Efficiency (%) | R2 | y-intercepts |
| Wild-type-sensitive | 3.39 | 97.2 | 0.998 | 37.705 |
| Mutant-resistant | 2.59 | 142.7 | 0.991 | 36.531 |
Table 5. List of D. rabiei isolates used for the in-vivo assay
| Isolate ID | QoI status | Location by state |
| AR-R001 | Resistant | Montana |
| AR-R002 | Resistant | Montana |
| AR-R003 | Resistant | Montana |
| AR-R004 | Resistant | North Dakota |
| AR-R005 | Resistant | North Dakota |
| AR 405 | Sensitive | Idaho |
| AR 407 | Sensitive | Idaho |
| AR 439 | Sensitive | Washington State |
| AR 411 | Sensitive | Idaho |
| AR 430 | Sensitive | Idaho |
Table 6.Cycling threshold values for D. pisi, D. lentis and A. alternata screened for G143A mutation using the SNP TaqMan assay
| Fungi species | Source of isolate | FAMa CT value | Cy5b CT value | Mean CT value |
| D. pisi | Pea seed | 0.00 | 17.69 | 17.69 |
| D. lentis | Lentil seed | 0.00 | 25.75 | 25.75 |
| A. alternata | Pea seed | 0.00 | 18.71 | 18.71 |
a Probe for resistant allele; b probe for sensitive allele
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more