ABSTRACTS
Tetraphenyltin in high yield has been prepared by the reaction of chlorobenzene, chlorotin (IV) and sodium metal in dry toluene characterized by Infra-red spectroscopy.
Introduction
Organic compounds that contains metal-carbon bond are called organometallic compounds. Organometallic compounds have been studied for nearly 200 years. Unique properties of these compounds have been used in many areas of life. The reactivity of organometallic compounds depends on the reduction potential of metal. For preparation and use, it is markedly observed that most reactivity requires low to moderate temperature and inert conditions like atmosphere and solvent. Generally the reactivity of these compounds seems like the ionic character of the carbon-metal bond, which may be estimated from the proton and carbon chemical shifts of methyl derivatives. % Ionic character of H3C–Metal as (CH3)2Hg < (CH3)2Cd < (CH3)2Zn < (CH3)2Mg < CH3L [1].
The first reported organometallic compounds were prepared by the reductive substitution of alkyl halides. Alkali metals have strong or moderate negative reduction potentials, with lithium and magnesium being the most reactive. Halide reactivity increases in the order: Cl < Br < I. Alkyl sodium and potassium compounds are not made in this way because Wurtz coupling of the alkyl moiety (giving R_R) tends to predominate. This can also be a problem when allyl or benzyl halides are converted to Grignard or lithium reagent [2].
There is an exceedingly extensive chemistry of the group four elements bound to carbon and some of the compounds, notably silicon-oxygen polymers and alkyl tin and lead compounds are of commercial importance. Essentially all the compounds are of the type M(IV) type. In the divalent state the only well established compounds are cyclopentadienyl tin alkyls or aryls of formula R2Sn are either transitory or non-existent, and the stable substances of this stoichiometry are linear or cyclic polymers of tetravalent tin.
For all the group four elements the compounds can generally be designated R4-nMXn where R is the alkyl or aryl and X can vary widely being H, Cl, O, COR, OR, NR2SR etc. For a given class of compounds those with C-Si and C-Ge bonds have higher thermal stability and lower reactivity than those with bonds to Sn and Pb [3].
There are four series of organotin compounds depending on the number of carbon-tin bonds. These series are designated as mono-, di-, tri-, and tetraorganotin compounds with the general formula: RnSn X4-n
Where
R = an alkyl or aryl group
Sn = the central tin atom in the oxidation state +4
X = a singly charged anion or an anionic organic group [4].
The ability of transition metals to form organo derivatives only begins to be appreciated properly during the nineteen fifties. Nonetheless, the organometallic compounds of transition metals now constitute an enormous, diversified field of chemistry, which is still expanding rapidly. It begins breadth by merging into the field of metal carbonyls and related compounds. They also differ in structure from that of non-transition organo-metal derivatives.
The transition metals form compounds in which there is metal to carbon sigma bond although pi bond in some cases may also be formed. More important, the unique characteristic of d orbital allow certain type of unsaturated hydrocarbons and some of their derivatives to be bound to metals in a non-classical manner to give molecules or ions with structures that have no counterpart elsewhere in chemistry. Not only is a wide range of organo compounds of different types are isolable, but also labile species play an important role in olefins, acetylene and their derivatives catalyzed by metal complexes [5].
Hypercoordinated Stannanes
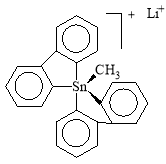 Tin compounds can also be coordinated to five atoms instead of the regular four unlike their carbon analogues. The stability of these hypercoordinated compounds is managed by electronegative substituent. In 2007 a stable organotin (all carbon pentaorganostannane) was reported at room-temperature (in argon) [4] in the form of lithium salt with the following structure.
Tin compounds can also be coordinated to five atoms instead of the regular four unlike their carbon analogues. The stability of these hypercoordinated compounds is managed by electronegative substituent. In 2007 a stable organotin (all carbon pentaorganostannane) was reported at room-temperature (in argon) [4] in the form of lithium salt with the following structure.
The geometry of the molecule is distorted trigonal bipyramidal. The carbon to tin bond lengths are 2.26Å apical, 2.17Å equatorial. These are larger than regular C–Sn bonds (2.14Å) which shows its hypervalent nature.
Biological Aspects
The chemistry of the organotin(IV) derivatives is being subject of study with growing interest, not only because of the environmental consequences of the widespread use of these compounds, but also as due to the increasingly importance of their medical assays for bactericide and antitumor purposes. In this respect, various triorganotins have been reported recently to be effective against mosquito larvae and adult mosquitoes responsible for malaria and yellow fever, and also some phenyltin derivatives display cardiovascular activity. In general, the structure-activity relationship in this kind of compounds is still subject of controversy, but it seems been established that, for instance, in the case of triorganotin carboxylates, those containing trans-O2SnC3 moieties exhibit a greater biocidal activity than those containing cis-O2SnC3.
Organotin compounds have been implicated as reproductive toxicants and endocrine disruptors primarily through studies in aquatic organisms, with little information available in mammals. Among the organotins, aryltins have been less studied than alkyltins. Extensive data is available on mammalian developmental and reproductive toxicity of one aryltin compound, triphenyltin (TPT), from toxicity studies conducted in connection with the registration of triphenyltin hydroxide (TPTH) as a pesticide and supporting publications from the open literature. Indications of adverse functional and morphological effects on the reproductive tract of rats were reported in a dose range of 1.4-20 mg/kg/d. Gonadal histopathology (both ovaries and testes) and infertility were affected at the higher doses, while reproductive-tract cancer, smaller litter sizes, and reproductive organ weights were affected at the lower end of the dose range. In vitro studies indicate the TPT can directly activate androgen receptor-mediated transcription and inhibit enzymes that are involved in steroid hormone metabolism. These data suggest that the aryltin TPT can be active as a reproductive toxicant in mammals and may be a human endocrine disruptor.
Organotins are one of the classes of compounds implicated as “endocrine disruptors” (Colborn & Clement, 1992) primarily on the basis of the finding of imposex in aquatic gastropods (Smith, 1981; Horiguchi et al., 1994). In these organisms, genetic females and parts of the male reproductive system, including the penis and vas deferens, superimposed on a normal female genital system. Implications for mammalian reproduction are less explored. There are no available epidemiological studies of the reproductive toxicity of organotins in humans or mammalian wildlife populations, so that mammalian reproductive toxicity information is limited to laboratory animals.
Among the organotins, a particularly large database on reproductive toxicity in laboratory animals is available through studies conducted for registration of triphenyltin hydroxide as a pesticide. Triphenyltin (TPT) came into use as a fungicide and matricide in the 1950s (HSDB, 1998). Currently, about 10 products containing TPT are registered for use as pesticides by the U.S. Environmental Protection Agency (U.S. EPA). Registration for TPT hydroxide was cancelled in California in 1983. TPT acetate and hydroxide were banned from use in the European Union (EU) in 2002 (Lo et al., 2003). This review presents and integrates information on reproductive toxicity from the triphenyltin hydroxide (TPTH) registration database made available through the Freedom of Information Act (FOIA), and includes papers on mammalian reproductive toxicity of other TPT salts. In this review, information from the pesticide registration data is presented in some detail since it is not available.
Estimates of exposure for total tin indicate that the main route in the general population is from food, about 4 mg/d. A national survey in the United States in 1982 reported 8.7-15 ïg tin/g in human adipose tissue (ATSDR, 1992). However, these estimates are based on outdated information, and much of the tin came from canned foods (inorganic tin) (ATSDR, 1992). The most recent information for TPT is from Japan (Tsuda et al., 1995). Duplicate portion studies indicated an intake of 0.7 ïg TPT/d in 1991 and 1992, and market basket surveys indicated intakes of 5.4 and 1.3 ïg TPT/d in 1991 and 1992, respectively. Analytical techniques are now becoming available to separate various forms of inorganic and organic tin which promise more accurate human exposure assessment.
 2Na + C6H5Cl C6H5Na + NaCl
2Na + C6H5Cl C6H5Na + NaCl
 4 C6H5Na +SnCl4 (C6H5)4Sn + 4NaCl
4 C6H5Na +SnCl4 (C6H5)4Sn + 4NaCl
Reagents required:
Special apparatus required:
Procedure
Fifteen grams of clean sodium chunks and 250 ml of dry toluene are placed in the flask. A thermometer and an argon inlet tube are inserted through one of the side arm of the flask. The other side arm is Stoppard. Insert the stirrer through the main mouth of the flask, taking care of that the stirring blades cannot hit the thermometer and that they are above the chunks of sodium. While stirring gently, and with a slow stream of argon flowing, heat the contents slowly to 105°. Then lower the stirrer so that the blades are about 1cm from the bottom of the flask and turn the stirrer on full power. It will be found necessary to increase the power input to the heating mental in order to keep the temperature at 105°. After about 10 min of vigorous stirring at 105°, remove the heating mantle from the flask. When the temperature has fallen to 99°, stop the stirrer and allow the flask to cool to room temperature. The sodium should now be in the form of fine sand. Stir the sodium gently to see if any of the particles have agglomerated. If so, the process must be repeated. If the sodium dispersion is not be used immediately, thoroughly flush the flask with the argon and tightly stopper it.
Using the heating mantle, heat the dispersion, with moderately vigorous stirring, to 45°. Attach a dropping funnel containing 35 ml of chlorobenzene to the unused side arm and add 2 to 3 ml of the chlorobenzene to the flask.
Notice: The flask should never contain more than 3 ml of un reacted chlorobenzene! If more than this amount is present, an uncontrollably vigorous reaction may take place, resulting in a fire.
Remove the heating mantle from the flask. The reaction should start, as evidenced by a rise in the temperature. If the reaction does not start at 45°, cautiously rise the temperature to 50° (no higher!). If the reaction starts at this temperature, the temperature may suddenly rise to as high as 55°, so be ready to cool the flask quickly with the kerosene bath. (If the reaction does not start at 50°, cool the flask to room temperature, cautiously hydrolyze the mixture with alcohol, and discard).
Temperature in access of 50° will not cause great harm at the beginning of the synthesis, but thereafter the temperature must be kept below 45°; keep the flask partially immersed in the kerosene bath and cool the kerosene bath by occasionally adding pieces of DRY Ice to it. The temperature of the reaction mixture may be held between 40 and 45° by adjusting the rate of addition of chlorobenzene.
After all the chlorobenzene has been added (about 1 to 2 hours), place a solution of 10ml of stannic chloride in 25ml of toluene in the dropping funnel, and, over a period of 30 min, add this solution to the reaction flask. During this addition, it is necessary to cool the flask so as to keep the temperature below 45°. The flask now be stored indefinitely (without protection from the air) until the tin tetra phenyl is extracted from the mixture.
Wipe the kerosene from the bottom of the flask, and, with moderate stirring, heat the mixture to incipient boiling and quickly filter through a sintered-glass funnel. It is best to keep most of the solid residue in the reaction flask. Cool the filtrate to room temperature and filter off the product on another sintered-glass funnel. Return the filtrate to the original flask and repeat the extraction two or three times until no more product precipitates on cooling the solution to room temperature. It is helpful to add another 100 ml of toluene to the mixture to reduce the necessary number of extractions. The final solution should be cooled in an ice bath before filtering. Suck the crystals of tin tetra phenyl as dry as possible on the filter and then let them air dry for 4 to 20 hours on a watch glass.
A yield of about 25 gm of material melting at 266 to 228° should be obtained. A pure product (melting at 299°) may be obtained by re crystallization from benzene or toluene.
Physical data for reported compounds are given in Table 1.
|
Compound # |
Empirical formula |
M.P (°C) |
Solubility |
% Yield |
|
1. |
(C6H5)4Sn |
110-112 |
Toluene, Ethanol, Chloroform |
72 |
IR is one of the most important spectroscopic methods used for qualitative and quantitative analysis. It is based on the fact that each compound has its own unique spectra and certain functional groups absorbat about the same wavelength even in different molecules. Its single most important use has been for the identification of organic compounds whose spectra are generally complex and provide numerous maxima and minima that are used for comparison purposes. Indeed in most instances the IR spectrum of the compounds especially of organic compounds provides a unique finger print, which is readily distinguished from the absorption pattern from all other compounds because only optical isomers absorb in the same way. Absorption of IR radiation is confined largely to molecular species for which small energy differences exist between various vibration and rotational states. As for as spectrum is concerned we see a prominent peak at 457cm-1, which indicates the formation of metal to carbon bond, which shows the formation of our product. For aliphatic CH peak appear at 3057 cm-1 .
References
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more