Topic Area: self-healing materials, shape memory polymers and flame retardant polymers
Emerging Polymer materials
Md Hossion Shovon
Introduction:
The ability of a nation to harness nature as well as its ability to cope up with the challenges posed by it is determined by its complete knowledge of materials and its ability to develop and produce them for various applications. Advanced Materials are at the heart of many technological developments that touch our lives. Electronic materials for communication and information technology, optical fibers, laser fibers sensors for the intelligent environment, energy materials for renewable energy and environment, light alloys for better transportation, materials for strategic applications and more. Advanced materials have a wider role to play in the upcoming future years because of its multiple uses and can be of a greater help for whole humanity. Emerging technologies are those technical innovations which represent progressive developments within a field for competitive advantage. List of currently emerging technologies, which contains some of the most prominent ongoing developments, advances, and Materials Science and Nanotechnology Innovations are Graphene, Fullerene, Conductive Polymers, Metamaterials, Nanomaterials: carbon nanotubes, Superalloy, Lithium-ion batteries, etc. Over the last decade, smart polymeric materials have been used in biochemical sciences in many ways. Since the term, “smart polymeric materials” encompasses a wide spectrum of different compounds with unique potential for biological applications,
Self-healing Polymers:
Self-healing polymers are a new class of smart materials that have the capability to repair themselves when they are damaged without the need for detection or repair by manual intervention of any kind. Increasing demand for petroleum feedstocks used to produce polymer and the need for polymeric materials with improved performance in challenging applications continue to drive the need for materials with extended lifetimes. One way to extend the lifetime of a material is to mitigate the mechanism leading to failure. In brittle polymers, failure occurs through crack formation and propagation (1,2) and the ability to repair these cracks when they are still very small will prevent further propagation thus extending the lifetime of the material. Emerging self-healing technologies designed to give polymeric materials the capability to arrest crack propagation at an early stage thereby preventing catastrophic failures will go a long way in helping to increase the scope of applications of these materials. With the need for autonomic repair of materials without external intervention thus evident, more recent research has focused on developing fully self-healing systems. One approach to the design of such systems employs the compartmentalization of a reactive healing agent, which is then incorporated into a composite material. Thus, when a crack propagates through the material, it causes the release of the healing agent from the compartment in which it is stored into the crack plane where it solidifies and repairs the material.
The first basic application of this approach consisted of an epoxy matrix with suspended glass capillaries filled with either cyanoacrylate or a two-part epoxy resin. When a crack propagated through the cured epoxy matrix, the glass capillaries were fractured and the cyanoacrylate monomer or the two-part epoxy generally referred to as healing agents, were released into the crack plane where they reacted and polymerized. A significant recovery of the mechanical properties of the samples after they were allowed to heal suggests that the cracked material was effectively repaired by the polymerized healing agent. Since the healing requires only crack propagation as the trigger for the healing mechanism, it represents a truly autonomic or self-healing material. While a successful demonstration of self-healing, the labor-intensive process of manually filling capillaries and distributing them evenly throughout the matrix make this approach unsuitable for scale-up.
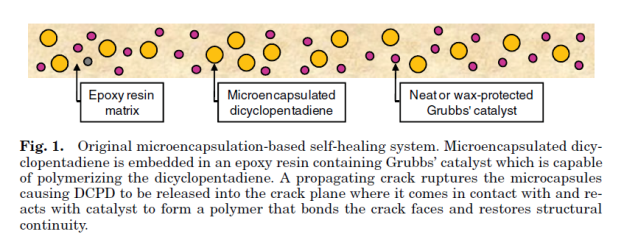
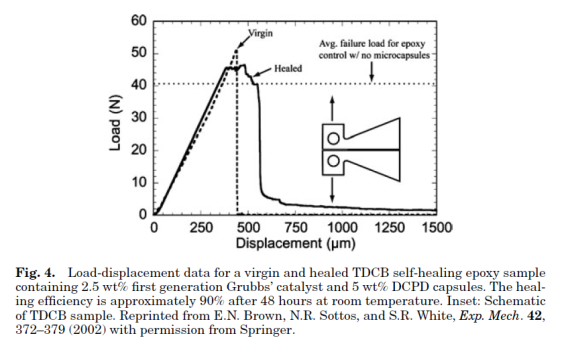
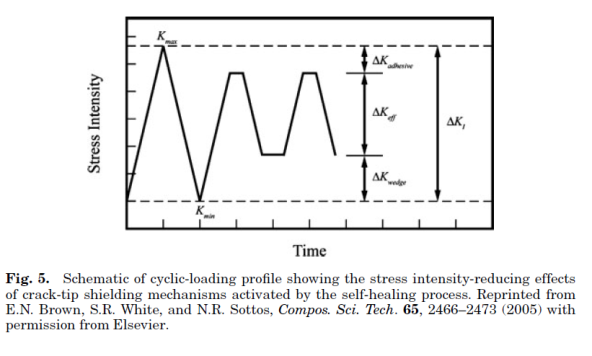
Shape-memory Polymers:
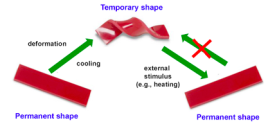
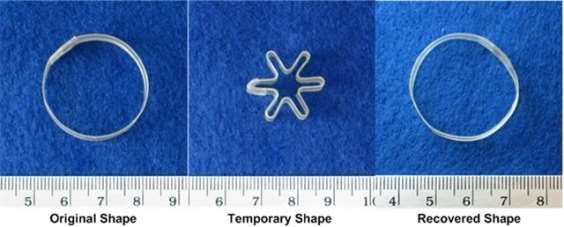
Shape-memory polymers are an emerging class of active polymers that have the dual-shape capability. They can change their shape in a predefined way from shape A to shape B when exposed to an appropriate stimulus. While shape B is given by the initial processing step, shape A is determined by applying a process called programming. The shape-memory research was initially founded on the thermally induced dual-shape effect. This concept has been extended to other stimuli by either indirect thermal actuation or direct actuation by addressing stimuli-sensitive groups on the molecular level. Finally, polymers are introduced that can be multifunctional. Besides their dual-shape capability, these active materials are biofunctional or biodegradable. Potential applications for such materials as active medical devices are highlighted. Shape-memory polymers are dual-shape materials belonging to the group of ‘actively moving’ polymers. They can actively change from a shape A to a shape B. Shape A is a temporary shape that is obtained by mechanical deformation and subsequent fixation of that deformation. This process also determines the change of shape shift, resulting in shape B, which is the permanent shape. In shape-memory polymers reported so far, heat or light has been used as the stimulus. Using irradiation with infrared light, application of electric fields, alternating magnetic fields, or immersion in water, indirect actuation of the shape memory effect has also been realized. The shape-memory effect only relies on the molecular architecture and does not require a specific chemical structure in the repeating units. Therefore, intrinsic material properties, e.g. mechanical properties, can be adjusted to the need of specific applications by variation of molecular parameters, such as the type of monomer or the comonomer ratio.
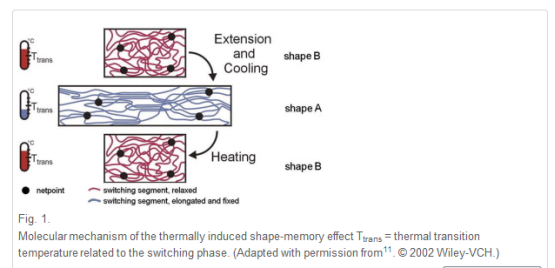
An example of a cross-linked polymer network synthesized by polyaddition of monofunctional monomers with low molecular weight or oligomeric cross-linkers has been realized in polyurethanes by the addition of trimethyl owl to the reaction mixture.
The reaction of tetra-functional silanes, working as net points, with oligomeric silanes, which work as spacers and to which two distinct benzoate-based mesogenic groups have been attached, results in a formation of a main-chain smectic-C elastomer38. In contrast to other liquid-crystalline elastomers, which display a shape-changing behavior and have been compared to shape-memory polymers recently, these elastomers have shape-memory properties. The cross-linking process during synthesis defines the permanent shape. The shape-memory effect is triggered by the thermal transition of the liquid-crystalline domains. In the programming process, the polymer network is heated to the isotropic state of the liquid crystalline domains, stretched or twisted, and then cooled below the clearing transition temperature of the smectic-C mesogens. Upon reheating over this clearing transition, the permanent shape can be recovered. In contrast to shape-changing liquid crystalline elastomer systems, these polymers display shape-memory behavior because the liquid crystalline moieties work as a switch. In shape-changing liquid-crystalline elastomers, the molecular movement of the single liquid crystals is converted into a macroscopic movement

Another class of thermoplastic shape memory polymers with Trans = Tg are polyesters. In copolyesters based on poly(ɛ-caprolactone) and poly (butylene terephthalate), the poly (butylene terephthalate) segments act as physical cross-linkers25. The shape-memory capability can also be added to a polymer using a polymer-analogous reaction. A polymer-analogous reaction is the application of a standard organic reaction (like the reduction of a ketone to an alcohol) to a polymer having several of these reactive groups. An example is the polymer-analogous reduction of a polyketone with NaBH4/THF, which results in a poly(ketone-co-alcohol). The polyketones are synthesized by late transition metal catalyzed polymerization of propene, hex-1-ene, or a mixture of propane and hex-1-ene with CO. The Tg of this polymer is directly related to the degree of reduction, which can be adjusted by the amount of NaBH4/THF. The most promising shape-memory material is a partly reduced poly (ethylene-co-propane-co-carbon oxide), which displayed a phase-separated morphology with hard microcrystalline ethylene/CO-rich segments within a softer amorphous polyketone ethylene-propene/CO-rich matrix. The crystalline domains of this material work as physical cross-linkers. This results in an elastic behavior above Tg because the glass transition temperature (Trans = Tg) is related to the switching phase. Partial reduction of the material allows control of Tg, which can be adjusted from below room temperature to 75°C.

Flame-retardant Polymers:
Fire-safe polymers are polymers that are resistant to degradation at high temperatures. There is need for fire-resistant polymers in the construction of small, enclosed spaces such as skyscrapers, boats, and airplane cabins. In these tight spaces, ability to escape in the event of a fire is compromised, increasing fire risk. In fact, some studies report that about 20% of victims of airplane crashes are killed not by the crash itself but by ensuing fires. Fire-safe polymers also find application as adhesives in aerospace materials, insulation for electronics and in military materials such as canvas tenting.
Some fire-safe polymers naturally exhibit an intrinsic resistance to decomposition, while others are synthesized by incorporating fire-resistant additives and fillers. Current research in developing fire-safe polymers is focused on modifying various properties of the polymers such as ease of ignition, rate of heat release, and the evolution of smoke and toxic gases. Standard methods for testing polymer flammability vary among countries; in the United States common fire tests include the UL 94 small-flame test, the ASTM E 84 Steiner Tunnel, and the ASTM E 622 National Institute of Standards and Technology (NIST) smoke chamber. Research on developing fire-safe polymers with more desirable properties is concentrated at the University of Massachusetts Amherst and at the Federal Aviation Administration where a long-term research program on developing fire-safe polymers was begun in 1995. The Center for UMass/Industry Research on Polymers (CUMIRP) was established in 1980 in Amherst, MA as a concentrated cluster of scientists from both academia and industry for the purpose of polymer science and engineering research.
Controlling the flammability of different materials has been a subject of interest since 450 B.C. when Egyptians attempted to reduce the flammability of wood by soaking it in potassium aluminum sulfate (alum). Research on fire-retardant polymers was bolstered by the need for new types of synthetic polymers in World War II. The combination of a halogenated paraffin and antimony oxide was found to be successful as a fire retardant for canvas tenting. Synthesis of polymers, such as polyesters, with fire retardant monomers were also developed around this time..


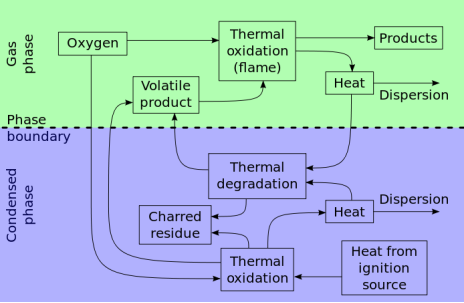
Additives are divided into two basic types depending on the interaction of the additive and polymer. Reactive flame retardants are compounds that are chemically built into the polymer. They usually contain heteroatoms. Additive flame retardants, on the other hand, are compounds that are not covalently bound to the polymer; the flame retardant and the polymer are just physically mixed together. Only a few elements are being widely used in this field: aluminum, phosphorus, nitrogen, antimony, chlorine, bromine, and in specific applications magnesium, zinc and carbon. One prominent advantage of the flame retardants (FRs) derived from these elements is that they are relatively easy to manufacture. The most important flame retardants systems used act either in the gas phase where they remove the high energy radical’s H and OH from the flame or in the solid phase, where they shield the polymer by forming a charred layer and thus protect the polymer from being attacked by oxygen and heat. Flame retardants based on bromine or chlorine, as well as a number of phosphorus compounds act chemically in the gas phase and are very efficient. Others only act in the condensed phase such as metal hydroxides (aluminum trihydrate, or ATH, magnesium hydroxide, or MDH, and boehmite), metal oxides and salts (zinc borate and zinc oxide, zinc hydroxystannate), as well as expandable graphite and some nanocomposites (see below). Phosphorus and nitrogen compounds are also effective in the condensed phase, and as they also may act in the gas phase, they are quite efficient flame retardants. Overviews of the main flame retardants families, their mode of action and applications are given in. Besides providing satisfactory mechanical properties and renewability, natural fibers are easier to obtain and much cheaper than man-made materials. Moreover, they are more environmentally friendly. Recent research focuses on application of different types of fire retardants during the manufacturing process as well as applications of fire retardants (especially intumescent coatings) at the finishing stage.
A good example for a very efficient phosphorus-based flame retardant system acting in the gas and condensed phases is aluminum diethyl phosphonate in conjunction with synergists such as melamine polyphosphate (MPP) and others. These phosphonates are mainly used to flame retard polyamides (PA) and polybutylene terephthalate (PBT) for flame retarded applications in electrical engineering/electronics (E&E).
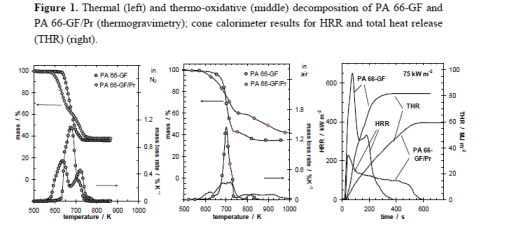
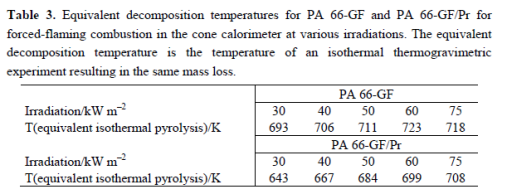
These are well illustrated by the investigations on glass fiber reinforced polyamide 66 flames retarded with red phosphorus (PA 66-GF/Pr), which demonstrate these charming characteristics Figure 1 shows the thermal and thermo-oxidative decomposition of PA 66-GF/Pr in comparison to PA 66-GF, as well as the performance in cone calorimeter experiments. For both materials, decomposition is characterized by at least three different processes, which strongly overlap for PA 66-GF and are clearly separated for PA 66-GF/Pr. Some decomposition processes are shifted to lower temperatures so that the decomposition region is broadened. There is only a small increase in thermal stability for the final decomposition step. Thermal decomposition changes from a one-step decomposition to a two-step decomposition characteristic. In fire tests, PA 66-GF/Pr is an effective charging material, achieving a clear reduction in THE and HRR in the cone calorimeter, as well as the highest self-extinction classification V-0 in the UL 94, whereas in the case of PA 66-GF all of the polymeric material is consumed so that only the glass fibers remain. Thermo-oxidative decomposition of PA 66 was concluded to occur in cone calorimeter experiments before ignition when a black skin is built up, and during afterglow after flame-out, when a further decrease in mass occurs accompanied by CO production. During the forced-flaming between ignition and flame-out, a stable flame rules out a major influence of oxygen on the decomposition during pyrolysis.
The mass loss after flaming combustion and the burning time are used to estimate an average effective pyrolysis temperature. This temperature was estimated by the necessary equivalent isothermal thermos gravimetry with the same mass loss in the burning time. This is a very rough estimation, of course, since the sample in the cone calorimeter, which is characterized by a temperature profile developing over time, is described by a constant temperature independent of place and time. However, since the specimens investigated were rather thin (2.8 mm) and contained inert filler, and because the fire residue was rather homogenous, the values summarized in Table 3 reasonably estimate the effect. The pyrolysis temperature for PA 66-GF is controlled by the decomposition temperature of the polymer and remains more or less constant for all irradiations used. The calculated temperature is higher than-but still close to-the temperature characteristic for the maximum mass loss rate in thermos gravimetry, and the temperature increases slightly with increasing irradiation. The PA 66 is consumed nearly completely by the pyrolysis zone running through the sample. The approximated pyrolysis temperature of PA 66-GF/Pr is characterized by the decomposition temperature of the first decomposition step and thus crucially lower than the temperatures concluded for PA 66-GF.
Summary:
The development and characterization of self-healing synthetic polymeric materials have been inspired by biological systems in which damage triggers an autonomic healing response. This is an emerging and fascinating area of research that could significantly extend the working life and safety of the polymeric components for a broad range of applications The past decade has witnessed remarkable advances in stimuli-responsive shape memory polymers (SMPs) with potential applications in biomedical devices, aerospace, textiles, civil engineering, bionics engineering, energy, electronic engineering, and household products. Shape memory polymer composites (SMPCs) have further enhanced and broadened the applications of shape memory polymers. In addition to reinforcement, SMPCs can enable or enhance thermal stimuli-active effects, novel shape memory effect, and new functions. Many thermal stimuli-responsive effects have been achieved such as electroactive effect, magnetic-active effect, water-active effect, and photoactive effect. The typical examples of novel shape memory effects are multiple shape memory effect, spatially controlled shape memory effect, and two-way shape memory effects. In addition, new functions of SMPCs have been observed and systemically studied such as stimuli-memory effect and self-healing. Flame retardancy of polymeric materials is conducted to provide fire protection to flammable consumer goods, as well as to mitigate fire growth in a wide range of fires. Incorporating flame-resistant additives into polymers became a common and relatively cheap way to reduce the flammability of polymers, while synthesizing intrinsically fire-resistant polymers has remained a more expensive alternative, although the properties of these polymers are usually more efficient at deterring combustion
References:
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more