ABSTRACT:
The aim of present work was to develop, characterisation, of stable ethosomal formulation as a carrier for transdermal delivery of paroxetine hydrochloride. To prepare this ethosome different concentration of soya lecithin and ethanol was taken. Vesicular size, polydispersity index, zeta potential, entrapment effiency were determined by photon correlation spectroscopy and ultracentrifugation techniques. The intro permeation study across human cadaver skin was done. Stability study was done on optimised F2 formulation. Vesicle size decrease as increase in the concentration of ethanol. Entrapment efficiency increase with increase in concentration of soya lecithin. The ethosome exhibit entrapment effiency of 40-64%. Invitro permeation study across human skin ethosome F2formulation showed higher transdermal flux 26.39%µg/cm2/hr. Release mechanism of Invitro permeation shows zero order drug release from formulation. In vivo pharmacodyanamic study F2 formulation showed significant immobility as compare to controlled group. Stability study result revealed no significant change found in size distribution was found for 90days. Our result indicates that the developed ethosomal system may be potential and safe to delivery paroxetine hydrochloride through transdermal deliverys.
INTRODUCTION:
In recent years the attraction of lipid vesicle use in delivery system for skin treatment is increasing (1, 2). Paroxetine hydrochloride (PXH) is a selective serotonin reuptake inhibitor. Commonly available in tablets and capsule dosage form, but oral administration have numbers of side effects as well as it undergoes extensive hepatic metabolism. Variation in plasma concentration and long term therapy leads to severe side effects (3).
To overcome these difficulties such as extensive hepatic first pass metabolism transdermal delivery is beneficial (4). The useful of transdermal delivery has been proved for some antidepressants (5,6). It is previously reported that significant increase delivery of drugs across the skin would be done by using an ethosomes as novel permeation enhancing carrier (7-10). Composition of ethosomes system mainly contains phospholipids, ethanol and water (12). Solubility and high encapsulation efficiency values for large range of lipophilic drugs can be obtain due to presence of ethanol. Ethanol may provide vesicles with soft flexible characteristics, which allow them to penetrate more simply into the deeper layers of the skin (13). The present aim focuses on the preparation and characterization of ethosomal formulation for PXH transdermal delivery. The aim of present study was to develop stable ethosomes carrier for transdermal delivery of PXH. The effect of ethanol and soya lecithin on the permeation of PXH through the human skin was evaluated.
Material and method:
Material:
Soya lecithin was purchased from Research Lab Mumbai. Ethanol was purchased from Loba chemical Mumbai. Cholesterol was purchased from Research Lab Fine Chem Industries, Mumbai. PEG-400 was purchased from Dipa Laboratory Chemicals. All materials and solvents used in this study are of analytical grade.
Preparation of ethosomes:
Soya lecithin and PXH, were dissolved in ethanol. Double distilled water was added slowly with a fine stream in above ethanol dispersion with constant mixing at 700 rpm on magnetic stirrer, in a well-sealed glass container. Mixing was continued for an additional 5 min. The system was kept at 300C throughout the preparation and was then left to cool at room temperature. (7, 8)
PHYSICAL CHARACTERISATION OF ETHOSOME:
Vesicles size distribution, polydispersity index and zeta potential
The vesicle size distribution, polydispersity index and zeta potential of vesicles was determined using photon correlation spectroscopy (Beckmann counter, Delsa Nano, USA). Formulation were diluted by 1/4th distilled water before measurement and measured three times at scattering angle of 900. The polydispersity index (PI) was used as a measurement of the width of the size distribution. PI less than 0.4 indicates a homogenous and monodisperse population. Zeta potential was measured as the particle electrophoretic mobility means of laser microelectrophoresis in a thermostated cell.
Entrapment efficiency (EE)
The entrapment capacity of PXH by ethosomal vesicles was determined by ultracentrifugation. Formulations were kept overnight at 4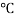 micro-centrifuge (Tarsons) 12000 rpm for 30 min. The supernatant was removed and drug amount was determined in both the sediment and the supernatant. The entrapment capacity was calculated as follows, [(T2C) /T] 100, where T is the total amount of drug that is detected both in the supernatant and sediment, and C is the amount of drug detected only in the supernatant.
micro-centrifuge (Tarsons) 12000 rpm for 30 min. The supernatant was removed and drug amount was determined in both the sediment and the supernatant. The entrapment capacity was calculated as follows, [(T2C) /T] 100, where T is the total amount of drug that is detected both in the supernatant and sediment, and C is the amount of drug detected only in the supernatant.
In vitro permeation study
Preparation of cadaver skin
Skin samples provided from Government Medical College and Hospital, Aurangabad. Obtain from breast reduction operation and subcutaneous fat was carefully trimmed and then rinse with normal saline, prepared skin was warped in aluminium foil and stored at -200c until use. (15)
Procedure
Invitro skin permeation studies were performed on a Franz diffusion cell with an effective diffusional area 0.785 cm2 and having receptor compartment volume of 15ml. The skin was brought to room temp and mounted with the donor compartment dry and open to the atmosphere. Initially, the donor compartment was empty and receiver compartment was filled with phosphate buffer ph 7.4. The receiver fluid was stirred with magnetic bead with the speed of 100rpm and the temperature was maintained at 37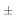 1
1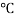 . The phosphate buffer pH 7.4 was replaced with the fresh one at every 30 min to stabilize the skin. It was found that the receiver solution should a negligible peak area after 3hr and beyond indicating complete stabilisation of skin. 5ml ethosomes formulation was placed into the donor compartment and sealed with paraffin film to provide occlusive condition. The sample where withdraw at regular interval for 10 hrs filtered through 0.45
. The phosphate buffer pH 7.4 was replaced with the fresh one at every 30 min to stabilize the skin. It was found that the receiver solution should a negligible peak area after 3hr and beyond indicating complete stabilisation of skin. 5ml ethosomes formulation was placed into the donor compartment and sealed with paraffin film to provide occlusive condition. The sample where withdraw at regular interval for 10 hrs filtered through 0.45 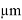 . membrane filter and analysed for drug amount by UV-Visible spectrophotometer at 294 nm.
. membrane filter and analysed for drug amount by UV-Visible spectrophotometer at 294 nm.
Permeation data analysis
The cumulative amount of penetrant, Q (g/cm2), which permeated the skin per unit surface area was plotted against time. The linear portion of the plot was taken as being the steady-state flux, (Js). The permeability coefficient (Kp) was calculated as:
Kp = Js/Cv
Where Cv is the concentration of penetrant in the donor solution.
Vesicle stability evaluation:
Stability of optimised ethosomes formulation was kept at room temp for 4 weeks. The measurement where conducted on of ethosomes that. Vesicle size, polydispersity index and zeta potential was measured at 1, 2, 3, 7, 14 and 21 days mean value where used for the analysed of the data.
2.5. In vivo Pharmacodynamic study
Approval to carry out pharmacodynamics studies was obtained (Institutional Animals Ethical Committee, approved the protocol). Forced Swim test (FST) and Locomotor Activity test (LAT) was used to evaluate antidepressant effect of the optimized F2 formulation. Rats of either sex weighing 250–300 g were kept under standard laboratory conditions (temperature 23-30oc).The rats were kept with free access to standard laboratory diet. Approximately 14 cm2 of abdominal side of rats skin was shaved on the in each group except group treated with marketed tablet formulation.
Rats were divided randomly into three groups each containing six animals. Group -1 was considered as a control. Group-2 was treated with oral tablet of PXH containing 1.40 mg/day and administered without anaesthesia by using simple poly-ethylene tube. Group-3 was treated with optimized F2 formulation applied transdermally containing 2 mg/day (equivalent to 0.60 mg/day) drug.
2.5.1. Force swim test
Rats were forced to swim in cylindrical glass tank (60 cm height X 30 cm in diameter) containing water after the administration of doses. Water was filled up to 40 cm height so they were swim without touching their hind limb or tail to bottom of the tank. On the 1st day of experiments, rats were forced to swim for 10 min. After 24 h, rats were re-exposed to forced swim for 5 min and animals were judged for immobility, climbing, and swimming. After a 5-min swim test, the rat was removed from the cylinder, dried and then returned to its home cage [29]. Porsolt, R.D., Bertin, A., Jalfre M. (1977). Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 229, 327–336.
2.5.2. Locomotor activity
Hyperactivity, functional roles of specific neurobiological and drugs potential psycho activity were discriminate by the locomotor activity study [30]. Locomotor activity was measured in the open-field test. The apparatus consisted of a square arena (200×200 cm), with a 50 cm height. The floor was divided into 30 equal squares. Animals were individually positioned in the centre of the arena and the activity was measured over 5 min. The open field was cleaned with isopropyl alcohol solution before behavioural testing to avoid possible bias due to odours and/or residues left by rats tested earlier. Also after each 3 animals apparatus was cleaned [31].
Result and discussion
Vesicle size, polydispersity index and zeta potential:
The vesicle mean diameters for all formulation are shown in Table 2. The result of photon correlation spectroscopy shows narrow peak for all formulation, which indicating that size of vesicle population is comparatively uniform in size. In accordance with other researcher, this decrease in the mean diameter of vesicle is due to the presence of ethanol (touitou et al., 2000). Higher concentration of ethanol produced lower vesicle size. Probably the ethanol causes the modification of the net charge of the system and confer it some degree of stearic stabilization that may finally lead to decrease in mean particle size (lasic et al., 1998). In the formulation the concentration of ethanol increases from 30-35% the significant decrease in vesicle size. On the other hand, it was observed that the increase in soya lecithin concentration resulted in increase in mean particle size. Small vesicle size is formed with the F2 formulation having a 1% of soya lecithin and 35% ethanol. Twice fold increase in soya lecithin concentration (1%-2%) resulted in two fold increase in ethosomes size (from 500nm- ). The charge of vesicles is important parameter that can influence both stability and skin vesicle interaction. Zeta potential value of all formulations shown in Table 2. The concentration of ethanol increase from 30-35% v/v resulting in increase in zeta potential values. Polydispersity index was determined as measure of homogeneity in formulation. Polydispersity index ≤ 0.3 indicate homogeneous population of ethosome vesicle in formulation. Polydispersity of all formulation shown in Table 2. Compare to all formulation F2 formulation showed less polydispersity index is 0.23 indicates homogeneous population of ethosome vesicles.
Entrapment efficiency
Entrapment efficiency of all formulation shown in Table 2. Entrapment efficiency of formulation containing of 1% soya lecithin and 30% (F1) ethanol was found to be 60%, which significantly increased to 64% when the amount of ethanol increases to 35% (F2) keeping the concentration of soya lecithin constant. Ethosomes formulation prepared with 1.5% soya lecithin and 30% ethanol (F3) exhibited 40% entrapment efficiency, which was increased to 45% (F4) respectively; keep the amount of soya lecithin constant. Formulation prepared with 2% soya lecithin and 30% ethanol (F5) showed 42% entrapment efficiency, which was increased to 61% when the concentration of ethanol increased to 35%(F6) respectively. These data supported by previous finding that solubility and high encapsulation efficiency values for large range of lipophilic drugs can be obtain due to presence of ethanol (13).From these results entrapment efficiency of formulation was observed due to increase in ethanol concentration.
Invitro permeation study
In vitro skin permeation experiment was performed using human cadaver skin showed that permeation was highest in F2 formulation as shown in Fig 1. Flux value of F2 formulation was significantly different when compared with other formulation (P≤0.05) as shown in Table 3. Highest flux value (———-) of F2 as compared to other formulation. These may be due to small vesicle size and high entrapment efficiency alone with high concentration of ethanol. These data supported by previous finding that ethanol interact with a lipid molecules of stratum corneum, resulting in reduction in the Tm of stratum corneum, increase in there fluidity. The intercalation of ethanol due to polar head group environment can result in increase in membrane permeability (16). It can also suggest that mixing of phospholipids with the stratum corneum lipid of the intercellular layers enhances the permeability of the skin (17). F2 formulation was selected as a optimized formulation from the vesicle size distribution, polydispersity index, zeta potential, drug entrapment efficiency, and in vitro permeation study results and considered for further study.
In vivo Pharmacodynamic study
Pharmacodynamic activity of ethosomes F2 formulation was compared with orally administered dose. Pharmacodynamic activity involved two tests. One was force swim test and other was locomotor activity. Force swim test is most widely used model for assessing the antidepressant activity. Total immobility period would decrease if high concentration of paroxetine hydrochloride reached target site.
Force swim test
Results of FST confirmed that there was significant reduction in total immobility period in seconds by treating the rats by transdermal ethosomal F2 formulation. There was significant (p<0.001) differences in total immobility period of control compared with paroxetine hydrochloride treated group as shown in Table 3. Ethosomes formulation significantly (p<0.001) reduced total immobility period by 15.69 ± 1.37sec than orally treated group.
Table 3. Results of forced swim test.
|
Treatment group |
Total immobility (sec) |
Mean |
||
|
Control |
170 |
168 |
171 |
169.33±3.35 |
|
Paroxetine hydrochloride oral administration |
110 |
112 |
118 |
115.35±3.95 |
|
Ethosome F2 transdermal application |
97 |
100 |
102 |
99.66±2.58 |
|
Values are expressed in mean ± SD, n = 3 , p value < 0.05 considered statistically significant |
Conclusion:
Ethosomal vesicles with appropriate size and maximum drug entrapment efficiency can be prepared. F2 formulation showed highest transdermal flux across human skin was composed of 1% soya lecithin, 35% ethanol and 2% cholesterol. In vivo pharmacodyanamic study of optimised formulation showed significant values compared to controlled group. Therefore, it can be concluded from the result of the study that ethosome formulation is potentially useful carrier for transdermal delivery of paroxetine hydrochloride
REFERANCES
Formulation of ethosome:
|
Batch |
Paroxetine HCL |
Soya lecithin |
cholesterol |
Ethanol |
Propylene glycol |
|
F1 |
2% |
1% |
2% |
30% |
0.5% |
|
F2 |
2% |
1% |
2% |
35% |
0.5% |
|
F3 |
2% |
1.5% |
1% |
30% |
0.5% |
|
F4 |
2% |
1.5% |
1% |
35% |
0.5% |
|
F5 |
2% |
2% |
2% |
30% |
0.5% |
|
F6 |
2% |
2% |
1% |
35% |
0.5% |
Table- 1
Evaluation of ethosome:
|
Batch |
Particle Size (nm) |
Polydispersity index |
Zeta potential(mV) |
EE (%) |
Transdermal Flux (µg/cm2/hr) |
Permeability coefficient (x10-2)(cm/hr) |
|
F1 |
500 |
0.473 |
-22.26 |
55 |
6.63 |
2.4 |
|
F2 |
694 |
0.23 |
-16.10 |
64 |
26.39 |
2.3 |
|
F3 |
1122 |
1.05 |
-25.48 |
40 |
9.12 |
0.013 |
|
F4 |
1222 |
0.685 |
-24.93 |
51 |
12.13 |
1.093 |
|
F5 |
1245 |
0.619 |
-28.46 |
42 |
3.34 |
0.029 |
|
F6 |
1311 |
0.949 |
-16.81 |
61 |
8.62 |
0.073 |
You have to be 100% sure of the quality of your product to give a money-back guarantee. This describes us perfectly. Make sure that this guarantee is totally transparent.
Read moreEach paper is composed from scratch, according to your instructions. It is then checked by our plagiarism-detection software. There is no gap where plagiarism could squeeze in.
Read moreThanks to our free revisions, there is no way for you to be unsatisfied. We will work on your paper until you are completely happy with the result.
Read moreYour email is safe, as we store it according to international data protection rules. Your bank details are secure, as we use only reliable payment systems.
Read moreBy sending us your money, you buy the service we provide. Check out our terms and conditions if you prefer business talks to be laid out in official language.
Read more